3D-Printed Coating of Extended-Release Matrix Tablets: Effective Tool for Prevention of Alcohol-Induced Dose Dumping Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Matrix Tablets with Tramadol Hydrochloride
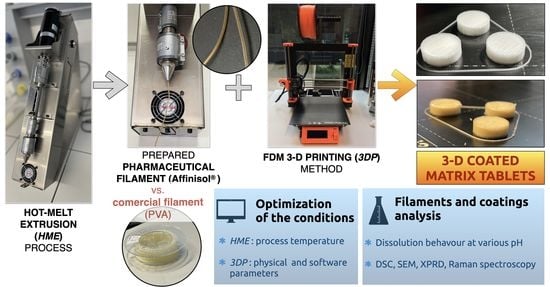
2.2.2. Preparation of the Filaments for 3D Coating
PVA
HPMC
2.2.3. 3D Coating of the Matrix Tablets
2.2.4. Scanning Electron Microscopy (SEM)
2.2.5. Differential Scanning Calorimetry (DSC)
2.2.6. Raman Spectroscopy
2.2.7. Powder X-ray Diffraction (PXRD)
2.2.8. In Vitro Drug Release Studies
Mathematical Evaluation of the Dissolution Data
2.2.9. Determination of the Released Amount of TH Using UV VIS Spectrometry
3. Results and Discussion
3.1. Preparation of the Filaments for 3D Coating
3.2. 3D Coating of the Matrix Tablets
- Preparation of the filament: choice of materials (or their combinations) that can melt and form a stable filament with required mechanical properties (elasticity and toughness).
- Ability to insert the filament into the 3D printer: successful preparation of the filament by HME does not guarantee the insertability into the 3D printer. Too fragile and smooth filaments are not utilizable in this regard.
- Mutual cohesion of the printed layers during (and also after) the 3D printing process. If the cohesion is insufficient, the model is unacceptably fragile.
3.3. SEM
3.4. DSC and Raman Characterization
3.5. Powder X-ray Diffraction (PXRD)
3.6. In Vitro Drug Release Studies
3.6.1. Acidic Medium pH 1.2: Dissolution Tests of the Uncoated Tablets and Tablets with PVA Coating
3.6.2. Acidic Medium pH 1.2:Dissolution Tests of the Uncoated Tablets and Tablets with Affinisol Coating
3.6.3. Alcoholic Medium (40% of Alcohol): Dissolution Tests of the Uncoated Tablets and Tablets with PVA Coating
3.6.4. Alcoholic Medium (40% of Alcohol): Dissolution Tests of the Uncoated Tablets and Tablets with Affinisol Coating
4. Conclusions
- The HME process was optimized for Affinisol, with the temperature of 150–160 °C being most effective.
- The procedures for 3D printing of Affinisol and PVA were optimized under the conditions given in Table 2.
- Based on the thermal and spectral analyses, no significant formation of the crystalline phase took place during the HME and 3D printing processes.
- The PVA coating did not influence the release mechanism of the model drug during the dissolution test in the acidic dissolution medium. The maximum released amount of the model drug from the formulation F1 (hydrophilic) was the same for the tablets without coating and for the tablets coated by one perimeter PVA. The coating by two and three perimeters of PVA decreased the maximum released amount of the model drug by ~10%. The maximum released amount of TH from the formulations F2 (lipophilic) and F3 (hydrophilic-lipophilic) was not influenced by the PVA coating.
- The PVA coating did not influence the release mechanism of the model drug in the alcoholic dissolution medium. The presence of Kollidon® SR caused the negative dose dumping effect and the release of the model drug was inhibited. The PVA coating did not prevent the release of the model drug during the first two hours of the dissolution testing in the alcoholic dissolution media.
- The Affinisol coating changed the release mechanism of the model drug in the acidic dissolution media. The coating reduced the model drug release at the beginning of the dissolution test.
- The Affinisol coating also changed the release mechanism of the model drug in the alcoholic dissolution media. At the beginning of the dissolution tests, no model drug was released for a significant time. Additionally, for the AFF-coated tablets, the Kollidon® SR caused the negative dose dumping effect. The Affinisol coating was, however, found to be suitable for the prevention of the dose dumping effect.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3DP | Three-dimensional printing |
| ADD | Alcohol-induced dose dumping |
| AFF | Affinisol |
| API | Active pharmaceutical substance |
| ASS | Absolute sum of squares |
| DSC | Differential scanning calorimetry |
| FDA | Food and Drug Administration |
| HME | Hot-melt extrusion |
| HPMC | Hypromellose |
| P | Perimeters |
| PE | Polyethylene |
| PVA | Polyvinyl alcohol |
| PXRD | Powder X-ray diffraction |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| TH | Tramadol hydrochloride |
References
- Fuenmayor, E.; Forde, M.; Healy, A.V.; Devine, D.M.; Lyons, J.G.; McConville, C.; Major, I. Material Considerations for Fused-Filament Fabrication of Solid Dosage Forms. Pharmaceutics 2018, 10, 44. [Google Scholar] [CrossRef] [Green Version]
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T. Serajuddin, Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J. Pharm. Sci. 2018, 107, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Tsintavi, E.; Rekkas, D.M.; Bettini, R. Partial tablet coating by 3D printing. Int. J. Pharm. 2020, 581, 119298. [Google Scholar] [CrossRef]
- Alruwaili, N.K.; Rizwanullah, M.; Bukhari, S.N.A.; Amir, M.; Ahmed, M.M.; Fazil, M. 3D Printing Technology in Design of Pharmaceutical Products. Curr. Pharm. Des. 2019, 24, 5009–5018. [Google Scholar] [CrossRef]
- Traynor, M.J.; Brown, M.; Pannala, A.; Beck, P.; Martin, G.P. Martin, Influence of alcohol on the release of tramadol from 24-h controlled-release formulations during in vitro dissolution experiments. Drug Dev. Ind. Pharm. 2008, 34, 885–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jedinger, N.; Schrank, S.; Mohr, S.; Feichtinger, A.; Khinast, J.; Roblegg, E. Alcohol dose dumping: The influence of ethanol on hot-melt extruded pellets comprising solid lipids. Eur. J. Pharm. Biopharm. 2015, 92, 83–95. [Google Scholar] [CrossRef]
- Jedinger, N.; Khinast, J.; Roblegg, E. The design of controlled-release formulations resistant to alcohol-induced dose dumping—A review. Eur. J. Pharm. Biopharm. 2014, 87, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Lochař, V.; Komersová, A.; Matzick, K.; Slezáková, B.; Bartoš, M.; Mužíková, J.; Haddouchi, S. The effect of alcohol on ionizing and non-ionizing drug release from hydrophilic, lipophilic and dual matrix tablets. Saudi Pharm. J. 2020, 28, 187–195. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Pereira, B.C.; Arafat, B.; Cieszynska, M.; Isreb, A.; Alhnan, M.A. Fabricating a shell-core delayed release tablet using dual FDM 3D printing for patient-centred therapy. Pharm. Res. 2017, 34, 427–437. [Google Scholar] [CrossRef]
- AlGahtani, M.S.; Mohammed, A.A.; Ahmad, J.; Saleh, E. Development of a 3D printed coating shell to control the drug release of Encapsulated immediate-release tablets. Polymers 2020, 12, 1395. [Google Scholar] [CrossRef]
- Raymond, C.; Rowe, P.; Sheskey, J.; Quinn, E.M. (Eds.) Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009; ISBN 9780853697923. [Google Scholar]
- Xu, X.; Zhao, J.; Wang, M.; Wang, L.; Yang, J. 3D Printed Polyvinyl Alcohol Tablets with Multiple Release Profiles. Sci. Rep. 2019, 9, 12487. [Google Scholar] [CrossRef] [PubMed]
- Salaoru, I.; Zhou, Z.; Morris, P.; Gibbons, G. Inkjet-printed Polyvinyl Alcohol Multilayers. J. Vis. Exp. 2017, 123, e55093. [Google Scholar] [CrossRef] [PubMed]
- Brochure Pharma Solutions AffinisolTM HPMC HME for Hot Melt Extrusion. Available online: https://www.pharma.dupont.com/content/dam/dupont/amer/us/en/nutrition-health/general/pharmaceuticals/documents/Download_Affinisol%20HPMC%20HME%20Brochure.pdf (accessed on 20 October 2021).
- Tagami, T.; Fukushige, K.; Ogawa, E.; Hayashi, N.; Ozeki, T. 3D Printing Factors Important for the Fabrication of Polyvinylalcohol Filament-Based Tablets. Biol. Pharm. Bull. 2017, 40, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; De Souza, L.R.; Litina, C.; Markaki, A.E.; Al-Tabbaa, A. Feasibility of Using 3D Printed Polyvinyl Alcohol (PVA) for Creating Self-Healing Vascular Tunnels in Cement System. Materials 2019, 12, 3872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2005, 57, 533–546. [Google Scholar] [CrossRef]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Huang, S.; O’Donnell, K.P.; Keen, J.M.; Rickard, M.; McGINITY, J.W.; Williams, R.O. A New Extrudable Form of Hypromellose: AFFINISOL™ HPMC HME. AAPS PharmSciTech 2016, 17, 106–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.S.; Solanki, N.; Serajuddin, A.T.M. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion, IV: AffinisolTM HPMC HME polymers. AAPS PharmSciTech 2016, 17, 148–157. [Google Scholar] [CrossRef] [PubMed]
- FDA (Food and Drug Administration). Guidance for Industry: Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs—General Considerations. Available online: https://www.gmp-compliance.org/files/guidemgr/UCM389370.pdf (accessed on 20 October 2021).
- PrimaselectTM PVA+ Description. Available online: https://primacreator.com/products/primaselect%E2%84%A2-pva?variant=61779045771 (accessed on 20 October 2021).
- AFFINISOLTM HPMC HME Brochure. Available online: https://www.pharma.dupont.com/pharmaceutical-brands/affinisol.html (accessed on 20 October 2021).
- Mužíková, J.; Komersová, A.; Lochař, V.; Vildová, L.; Vošoustová, B.; Bartoš, M. Comparative evaluation of the use of dry binders in a physical mixture or as a coprocessed dry binder in matrix tablets with extended drug release. Acta Pharm. 2018, 68, 295–311. [Google Scholar] [CrossRef] [Green Version]
- Komersová, A.; Lochař, V.; Myslíková, K.; Mužíková, J.; Bartoš, M. Formulation and dissolution kinetics study of hydrophilic matrix tablets with tramadol hydrochloride and different co-processed dry binders. Eur. J. Pharm. Sci. 2016, 95, 36–45. [Google Scholar] [CrossRef]
- Khatri, P.; Katikaneni, P.; Desai, D.; Minko, T. Evaluation of Affinisol® HPMC polymers for direct compression proces applications. J. Drug Deliv. Sci. Technol. 2018, 47, 461–467. [Google Scholar] [CrossRef]
- Rozo, J.I.J.; Zarow, A.; Zhou, B.; Pinal, R.; Iqbal, Z.; Romañach, R.J. Complementary near-infrared and Raman imaging of pharmaceutical thin films. J. Pharm. Sci. 2011, 100, 4888–4895. [Google Scholar] [CrossRef] [PubMed]
- Prasad, E.; Islam, M.T.; Goodwin, D.J.; Megarry, A.J.; Halbert, G.W.; Florence, A.J.; Robertson, J. Development of a Hot-Melt Extrusion (HME) process to produce drug loaded Affinisol™ 15LV filaments for Fused Filament Fabrication (FFF) 3D printing. Addit. Manuf. 2019, 29, 100776. [Google Scholar] [CrossRef]
- Meena, T.; Parikh, S.S.; Gupta, A.T.M. Serajuddin, Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion, II: Cellulosic polymers. J. Excip. Food Chem. 2014, 5, 46–55. [Google Scholar]
- Liu, P.; Chen, W.; Liu, C.; Tian, M.; Liu, P. A novel poly(vinyl alcohol)/poly(ethylene glycol) scaffold for tissue engineering with a unique bimodal open-celled structure fabricated using supercritical fluid foaming. Sci. Rep. 2019, 9, 9534. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.-M.; Yang, C.-C.; Hu, C.-C.; Pai, Y.-L.; Lue, S.J. Novel quaterrnized polyvinyl alcohol/ quaterrnized chitosan nano-composite as an effective hydroxide-conducting electrolyte. J. Membr. Sci. 2015, 485, 17–29. [Google Scholar] [CrossRef]
- Smyj, R.; Wang, X.-P.; Han, F. Tramadol Hydrochloride. Profiles Drug Subst. Excip. Relat. Methodol. 2013, 38, 463–494. [Google Scholar] [CrossRef]



















| Formulation | F1 | F2 | F3 |
|---|---|---|---|
| Tramadol hydrochloride | 20 | 20 | 20 |
| Kollidon® SR | 50 | - | 25 |
| Compritol® 888 ATO | - | 50 | 25 |
| Kolliwax® S | 5 | 5 | 5 |
| Prosolv® SMCC 90 | 25 | 25 | 25 |
| Material | Nozzle Temperature | Bed Temperature | Notes |
|---|---|---|---|
| AFF | 205 °C | 95 °C | Very fragile imprints. Requires cautious removal from the bed. Lower temperature causes worse cohesion of the layers. |
| PVA | 190 °C | 50–80 °C | Higher temperature of the nozzle causes shape deformation |
| 3D Coating Times | |||
|---|---|---|---|
| Number of Coatings | Thickness P1 | Thickness P2 | Thickness P3 |
| 1 | 3 min | 3 min | 4 min |
| 7 | 19 min | 23 min | 30 min |
| 10 | 27 min | 32 min | 42 min |
| 50 | 133 min | 159 min | 208 min |
| Weibull Model | |||||
|---|---|---|---|---|---|
| (kw ± SD) × 103 (min−ß) | A∞ ± SD (%) | ß ± SD | ASS | R2 | |
| Without coating | 25.57 ± 1.57 | 113.6 ± 2.89 | 0.63 ± 0.01 | 112 | 0.9974 |
| PVA I | 29.06 ± 2.13 | 107.8 ± 2.84 | 0.62 ± 0.02 | 153 | 0.9962 |
| PVA II | 0.42 ± 0.12 | 91.9 ± 1.98 | 1.29 ± 0.05 | 311 | 0.9940 |
| PVA III | 0.04 ± 0.01 | 85.3 ± 1.25 | 1.69 ± 0.07 | 313 | 0.9940 |
| The First-Order Kinetic Model | ||||
|---|---|---|---|---|
| (k1 ± SD) × 103 (min−1) | A∞ ± SD (%) | ASS | R2 | |
| Without coating | 5.84 ± 0.2 | 103.6 ± 0.99 | 726 | 0.9865 |
| PVA I | 2.94 ± 0.1 | 109.9 ± 1.87 | 708 | 0.9900 |
| PVA II | 5.75 ± 0.2 | 114.1 ± 2.88 | 1400 | 0.9820 |
| PVA III | 2.82 ± 0.2 | 113.9 ± 3.74 | 2507 | 0.9691 |
| Weibull Model | |||||
|---|---|---|---|---|---|
| (kw ± SD) × 103 (min−ß) | A∞ ± SD (%) | ß ± SD | ASS | R2 | |
| Without coating | 14.37 ± 2.10 | 107.1 ± 1.43 | 0.81 ± 0.03 | 436 | 0.9919 |
| PVA I | 0.61 ± 0.10 | 102.3 ± 0.79 | 1.30 ± 0.03 | 203 | 0.9971 |
| PVA II | 0.19 ± 0.05 | 102.7 ± 0.67 | 1.51 ± 0.04 | 213 | 0.9973 |
| PVA III | 0.05 ± 0.01 | 101.6 ± 0.54 | 1.78 ± 0.05 | 193 | 0.9976 |
| Weibull Model | |||||
|---|---|---|---|---|---|
| (kw ± SD) × 103 (min−ß) | A∞ ± SD (%) | ß ± SD | ASS | R2 | |
| Without coating | 23.28 ± 2.1 | 100.4 ± 2.19 | 0.68 ± 0.02 | 204 | 0.9957 |
| PVA I | 0.87 ± 0.2 | 99.4 ± 0.92 | 1.23 ± 0.03 | 201 | 0.9969 |
| PVA II | 0.21 ± 0.6 | 99.0 ± 0.91 | 1.46 ± 0.04 | 253 | 0.9964 |
| PVA III | 0.22 ± 0.04 | 99.3 ± 0.71 | 1.46 ± 0.03 | 162 | 0.9976 |
| Weibull Model | |||||
|---|---|---|---|---|---|
| (kw ± SD) × 103 (min−ß) | A∞ ± SD (%) | ß ± SD | ASS | R2 | |
| Without coating | 25.57 ± 1.57 | 113.6 ± 2.89 | 0.63 ± 0.01 | 112 | 0.9974 |
| Affinisol I | 1.47 ± 0.02 | 94.5 ± 1.74 | 3.28 ± 0.14 | 365 | 0.9939 |
| Affinisol II | 1.48 ± 0.03 | 75.5 ± 1.57 | 3.38 ± 0.18 | 357 | 0.9911 |
| Weibull Model | |||||
|---|---|---|---|---|---|
| (kw ± SD) × 103 (min−ß) | A∞ ± SD (%) | ß ± SD | ASS | R2 | |
| Without coating | 14.37 ± 2.10 | 107.1 ± 1.43 | 0.89 ± 0.03 | 436 | 0.9919 |
| Affinisol I | 0.02 ± 0.01 | 93.9 ± 1.97 | 1.75 ± 0.09 | 595 | 0.9905 |
| Affinisol II | 0.02 ± 0.01 | 91.2 ± 1.89 | 2.09 ± 0.12 | 613 | 0.9899 |
| Weibull Model | |||||
|---|---|---|---|---|---|
| (kw ± SD) × 103 (min−ß) | A∞ ± SD (%) | ß ± SD | ASS | R2 | |
| Without coating | 23.28 ± 2.1 | 100.4 ± 2.19 | 0.68 ± 0.02 | 204 | 0.9957 |
| Affinisol I | 2.16 ± 0.04 | 95.6 ± 1.19 | 1.97 ± 0.08 | 338 | 0.9951 |
| Affinisol II | 1.77 ± 0,02 | 91.5 ± 1.01 | 2.33 ± 0.06 | 153 | 0.9974 |
| Weibull Model | |||||
|---|---|---|---|---|---|
| (kw ± SD) × 103 (min−ß) | A∞ ± SD (%) | ß ± SD | ASS | R2 | |
| Without coating | 59.07 ± 5.1 | 36.62 ± 1.45 | 0.52 ± 0.03 | 38 | 0.9907 |
| PVA I | 7.62 ± 3.31 | 38.86 ± 1.14 | 0.92 ± 0.09 | 388 | 0.9580 |
| PVA II | 6.12 ± 2.44 | 38.34 ± 1.06 | 0.95 ± 0.08 | 289 | 0.9679 |
| PVA III | 4.23 ± 1.18 | 44.41 ± 2.15 | 0.91 ± 0.06 | 137 | 0.9858 |
| Weibull Model | |||||
|---|---|---|---|---|---|
| (kw ± SD) × 103 (min−ß) | A∞ ± SD (%) | ß ± SD | ASS | R2 | |
| Without coating | 4.37 ± 1.06 | 102.7 ± 1.41 | 1.02 ± 0.05 | 683 | 0.9881 |
| PVA I | 0.32 ± 0.05 | 100.5 ± 0.45 | 1.43 ± 0.03 | 94 | 0.9987 |
| PVA II | 0.04 ± 0.05 | 100.3 ± 0.25 | 1.78 ± 0.02 | 42 | 0.9995 |
| PVA III | 0.09 ± 0.04 | 98.7 ± 0.98 | 1.64 ± 0.07 | 560 | 0.9925 |
| Weibull Model | |||||
|---|---|---|---|---|---|
| (kw ± SD) × 103 (min−ß) | A∞ ± SD (%) | ß ± SD | ASS | R2 | |
| Without coating | 1.81 ± 0.04 | 99.9 ± 0.15 | 0.58 ± 0.02 | 306 | 0.9875 |
| PVA I | 1.39 ± 0.25 | 98.3 ± 4.17 | 1.05 ± 0.04 | 63 | 0.9971 |
| PVA II | 1.96 ± 0.38 | 97.9 ± 2.75 | 1.03 ± 0.04 | 65 | 0.9973 |
| PVA III | 0.96 ± 0.15 | 98.7 ± 0.88 | 1.11 ± 0.01 | 4 | 0.9998 |
| Weibull Model | |||||
|---|---|---|---|---|---|
| (kw ± SD) × 103 (min−ß) | A∞ ± SD (%) | ß ± SD | ASS | R2 | |
| Without coating | 64.67 ± 11.7 | 43.51 ± 1.14 | 0.59 ± 0.05 | 214 | 0.9695 |
| Affinisol I | 0.14 ± 0.12 | 30.73 ± 3.49 | 1.38 ± 0.16 | 170 | 0.9648 |
| Affinisol II | 0.003 ± 0.02 | 50.29 ± 3.05 | 1.89 ± 0.10 | 70 | 0.9937 |
| Weibull Model | |||||
|---|---|---|---|---|---|
| (kw ± SD) × 103 (min−ß) | A∞ ± SD (%) | ß ± SD | ASS | R2 | |
| Without coating | 4.42 ± 1.06 | 103.1 ± 1.42 | 1.01 ± 0.05 | 677 | 0.9883 |
| Affinisol I | 0.007 ± 0.004 | 94.6 ± 1.64 | 1.91 ± 0.09 | 433 | 0.9933 |
| Affinisol II | 0.002 ± 0.001 | 89.5 ± 2.10 | 2.39 ± 0.13 | 535 | 0.9906 |
| Weibull Model | |||||
|---|---|---|---|---|---|
| (kw ± SD) × 103 (min−ß) | A∞ ± SD (%) | ß ± SD | ASS | R2 | |
| Without coating | 23.28± 2.05 | 110.4 ± 2.19 | 0.68 ± 0.02 | 204 | 0.9957 |
| Affinisol I | 0.006 ± 0.002 | 95.6 ± 1.19 | 1.97 ± 0.08 | 338 | 0.9951 |
| Affinisol II | 0.004 ± 0.001 | 91.53 ± 1.01 | 2.33 ± 0.06 | 153 | 0.9974 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalická, B.; Matzick, K.; Komersová, A.; Svoboda, R.; Bartoš, M.; Hromádko, L. 3D-Printed Coating of Extended-Release Matrix Tablets: Effective Tool for Prevention of Alcohol-Induced Dose Dumping Effect. Pharmaceutics 2021, 13, 2123. https://doi.org/10.3390/pharmaceutics13122123
Skalická B, Matzick K, Komersová A, Svoboda R, Bartoš M, Hromádko L. 3D-Printed Coating of Extended-Release Matrix Tablets: Effective Tool for Prevention of Alcohol-Induced Dose Dumping Effect. Pharmaceutics. 2021; 13(12):2123. https://doi.org/10.3390/pharmaceutics13122123
Chicago/Turabian StyleSkalická, Barbora, Kevin Matzick, Alena Komersová, Roman Svoboda, Martin Bartoš, and Luděk Hromádko. 2021. "3D-Printed Coating of Extended-Release Matrix Tablets: Effective Tool for Prevention of Alcohol-Induced Dose Dumping Effect" Pharmaceutics 13, no. 12: 2123. https://doi.org/10.3390/pharmaceutics13122123
APA StyleSkalická, B., Matzick, K., Komersová, A., Svoboda, R., Bartoš, M., & Hromádko, L. (2021). 3D-Printed Coating of Extended-Release Matrix Tablets: Effective Tool for Prevention of Alcohol-Induced Dose Dumping Effect. Pharmaceutics, 13(12), 2123. https://doi.org/10.3390/pharmaceutics13122123