3D Printed Buccal Films for Prolonged-Release of Propranolol Hydrochloride: Development, Characterization and Bioavailability Prediction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
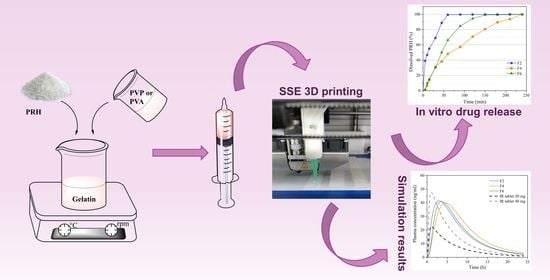
2.2.1. Preparation of 3D Printed Films
2.2.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Mechanical Properties
Tensile Test
Microindentation
2.2.5. Mucoadhesive Properties
2.2.6. Field Emission Scanning Electron Microscope (FESEM)
2.2.7. Drug Content Uniformity
2.2.8. In Vitro Drug Release
2.2.9. Drug Release Kinetics
2.2.10. Physiologically Based Simulations
2.2.11. Statistical Analysis of the Experimental Results
3. Results and Discussion
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2. Differential Scanning Calorimetry (DSC)
3.3. Mechanical Properties
3.3.1. Tensile Test
3.3.2. Microindentation
3.4. Mucoadhesive Properties
3.5. Field Emission Scanning Electron Microscope (FESEM)
3.6. Drug Content Uniformity
3.7. In Vitro Drug Release
3.8. Drug Release Kinetics
3.9. Physiologically Based Simulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Wang, H.; Xu, X.; Yang, G. Strategies and mechanisms to improve the printability of pharmaceutical polymers Eudragit® EPO and Soluplus®. Int. J. Pharm. 2021, 599, 120410. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Etxabide, A.; Long, J.; Guerrero, P.; de la Caba, K.; Seyfoddin, A. 3D printed lactose-crosslinked gelatin scaffolds as a drug delivery system for dexamethasone. Eur. Polym. J. 2019, 114, 90–97. [Google Scholar] [CrossRef]
- Elkasabgy, N.A.; Mahmoud, A.A.; Maged, A. 3D printing: An appealing route for customized drug delivery systems. Int. J. Pharm. 2020, 588, 119732. [Google Scholar] [CrossRef]
- Elkasabgy, N.A.; Mahmoud, A.A. Fabrication Strategies of Scaffolds for Delivering Active Ingredients for Tissue Engineering. AAPS PharmSciTech 2019, 20, 256. [Google Scholar] [CrossRef]
- Reddy, R.D.P.; Sharma, V. Additive manufacturing in drug delivery applications: A review. Int. J. Pharm. 2020, 589, 119820. [Google Scholar] [CrossRef]
- Eleftheriadis, G.; Ritzoulis, C.; Bouropoulos, N.; Tzetzis, D.; Andreadis, D.A.; Boetker, J.; Rantanen, J.; Fatouros, D.G. Unidirectional drug release from 3D printed mucoadhesive buccal films using FDM technology: In vitro and ex vivo evaluation. Eur. J. Pharm. Biopharm. 2019, 144, 180–192. [Google Scholar] [CrossRef]
- Preis, M.; Breitkreutz, J.; Sandler, N. Perspective: Concepts of printing technologies for oral film formulations. Int. J. Pharm. 2015, 494, 578–584. [Google Scholar] [CrossRef]
- Ouazib, F.; Mokhnachi, N.B.; Haddadine, N.; Barille, R. Role of polymer/polymer and polymer/drug specific interactions in drug delivery systems. J. Polym. Eng. 2019, 39, 534–544. [Google Scholar] [CrossRef]
- Ahmady, A.; Abu Samah, N.H. A review: Gelatine as a bioadhesive material for medical and pharmaceutical applications. Int. J. Pharm. 2021, 608, 121037. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)–A Versatile Polymer for Biomedical and Beyond Medical Applications. Polym. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Vecchi, C.F.; Cesar, G.B.; De Souza, P.R.; Caetano, W.; Bruschi, M.L. Mucoadhesive polymeric films comprising polyvinyl alcohol, polyvinylpyrrolidone, and poloxamer 407 for pharmaceutical applications. Pharm. Dev. Technol. 2020, 26, 138–149. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Dvořák, J.; Rosiak, N.; Tykarska, E.; Szymańska, E.; Winnicka, K.; Ruchała, M.A.; Cielecka-Piontek, J. Buccal Resveratrol Delivery System as a Potential New Concept for the Periodontitis Treatment. Pharmaceutics 2021, 13, 417. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Streisand, J.B. Oral Mucosal Drug Delivery. Clin. Pharmacokinet. 2002, 41, 661–680. [Google Scholar] [CrossRef]
- Adrover, A.; Di Muzio, L.; Trilli, J.; Brandelli, C.; Paolicelli, P.; Petralito, S.; Casadei, M.A. Enhanced Loading Efficiency and Mucoadhesion Properties of Gellan Gum Thin Films by Complexation with Hydroxypropyl-β-Cyclodextrin. Pharmaceutics 2020, 12, 819. [Google Scholar] [CrossRef]
- Bernardi, S.; Anderson, A.; Macchiarelli, G.; Hellwig, E.; Cieplik, F.; Vach, K.; Al-Ahmad, A. Subinhibitory Antibiotic Concentrations Enhance Biofilm Formation of Clinical Enterococcus faecalis Isolates. Antibiotics 2021, 10, 874. [Google Scholar] [CrossRef]
- Davoudi, Z.; Rabiee, M.; Houshmand, B.; Eslahi, N.; Khoshroo, K.; Rasoulianboroujeni, M.; Tahriri, M.; Tayebi, L. Development of chitosan/gelatin/keratin composite containing hydrocortisone sodium succinate as a buccal mucoadhesive patch to treat desquamative gingivitis. Drug Dev. Ind. Pharm. 2017, 44, 40–55. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, S.; Bianchi, S.; Tomei, A.R.; Continenza, M.A.; Macchiarelli, G. Microbiological and SEM-EDS Evaluation of Titanium Surfaces Exposed to Periodontal Gel: In Vitro Study. Materials 2019, 12, 1448. [Google Scholar] [CrossRef] [Green Version]
- Salehi, S.; Boddohi, S. Design and optimization of kollicoat ® IR based mucoadhesive buccal film for co-delivery of rizatriptan benzoate and propranolol hydrochloride. Mater. Sci. Eng. C 2018, 97, 230–244. [Google Scholar] [CrossRef]
- Shiledar, R.R.; Tagalpallewar, A.A.; Kokare, C.R. Formulation and in vitro evaluation of xanthan gum-based bilayered mucoadhesive buccal patches of zolmitriptan. Carbohydr. Polym. 2014, 101, 1234–1242. [Google Scholar] [CrossRef]
- Kurćubić, I.; Vajić, U.-J.; Cvijić, S.; Crevar-Sakač, M.; Bogavac-Stanojević, N.; Miloradović, Z.; Mihajlović-Stanojević, N.; Ivanov, M.; Karanović, D.; Jovović, D.; et al. Mucoadhesive buccal tablets with propranolol hydrochloride: Formulation development and in vivo performances in experimental essential hypertension. Int. J. Pharm. 2021, 610, 121266. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.; Simoes, S. Oral films: Current status and future perspectives. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Went, T.R.; Sultan, W.; Sapkota, A.; Khurshid, H.; Qureshi, I.A.; Jahan, N.; Tara, A.; Win, M.; Wiltshire, D.A.; Kannan, A.; et al. A Systematic Review on the Role of Βeta-Blockers in Reducing Cardiac Arrhythmias in Long QT Syndrome Subtypes 1-3. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Garakani, A.; Murrough, J.W.; Freire, R.C.; Thom, R.P.; Larkin, K.; Buono, F.D.; Iosifescu, D.V. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef]
- Shanker, V. Essential tremor: Diagnosis and management. BMJ 2019, 366, l4485. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, M.; Tomić, N.; Cvijić, S.; Stojanović, D.; Ibrić, S.; Uskoković, P. Mucoadhesive Gelatin Buccal Films with Propranolol Hydrochloride: Evaluation of Mechanical, Mucoadhesive, and Biopharmaceutical Properties. Pharmaceutics 2021, 13, 273. [Google Scholar] [CrossRef]
- Abruzzo, A.; Nicoletta, F.P.; Dalena, F.; Cerchiara, T.; Luppi, B.; Bigucci, F. Bilayered buccal films as child-appropriate dosage form for systemic administration of propranolol. Int. J. Pharm. 2017, 531, 257–265. [Google Scholar] [CrossRef]
- Elmadani, A.A.; Radović, I.; Tomić, N.Z.; Petrović, M.; Stojanović, D.; Heinemann, R.J.; Radojević, V. Hybrid denture acrylic composites with nanozirconia and electrospun polystyrene fibers. PLoS ONE 2019, 14, e0226528. [Google Scholar] [CrossRef] [Green Version]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- King, R. Elastic analysis of some punch problems for a layered medium. Int. J. Solids Struct. 1987, 23, 1657–1664. [Google Scholar] [CrossRef]
- Cevher, E.; Taha, M.A.; Orlu, M.; Araman, A. Evaluation of Mechanical and Mucoadhesive Properties of Clomiphene Citrate Gel Formulations Containing Carbomers and Their Thiolated Derivatives. Drug Deliv. 2008, 15, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Ashri, L.Y.; Ela, A.E.S.F.A.E.; Ibrahim, M.A.; Alshora, D.H.; Naguib, M.J. Optimization and evaluation of chitosan buccal films containing tenoxicam for treating chronic periodontitis: In vitro and in vivo studies. J. Drug Deliv. Sci. Technol. 2020, 57, 101720. [Google Scholar] [CrossRef]
- Chen, J.; Duan, H.; Pan, H.; Yang, X.; Pan, W. Two types of core/shell fibers based on carboxymethyl chitosan and Sodium carboxymethyl cellulose with self-assembled liposome for buccal delivery of carvedilol across TR146 cell culture and porcine buccal mucosa. Int. J. Biol. Macromol. 2019, 128, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krkobabić, M.; Medarević, D.; Pešić, N.; Vasiljević, D.; Ivković, B.; Ibrić, S. Digital Light Processing (DLP) 3D Printing of Atomoxetine Hydrochloride Tablets Using Photoreactive Suspensions. Pharmaceutics 2020, 12, 833. [Google Scholar] [CrossRef] [PubMed]
- Heade, J.; McCartney, F.; Chenlo, M.; Marro, O.; Severic, M.; Kent, R.; Bleiel, S.; Alvarez, C.; Griffin, B.; Brayden, D. Synthesis and In Vivo Evaluation of Insulin-Loaded Whey Beads as an Oral Peptide Delivery System. Pharmaceutics 2021, 13, 656. [Google Scholar] [CrossRef]
- Al-Ani, E.; Hill, D.; Doudin, K. Chlorhexidine Mucoadhesive Buccal Tablets: The Impact of Formulation Design on Drug Delivery and Release Kinetics Using Conventional and Novel Dissolution Methods. Pharmaceuticals 2021, 14, 493. [Google Scholar] [CrossRef]
- Kurcubic, I.; Cvijic, S.; Filipcev, B.; Ignjatovic, J.; Ibric, S.; Djuris, J. Development of propranolol hydrochloride bilayer mucoadhesive buccal tablets supported by in silico physiologically-based modeling. React. Funct. Polym. 2020, 151, 104587. [Google Scholar] [CrossRef]
- Mishra, R.; Varshney, R.; Das, N.; Sircar, D.; Roy, P. Synthesis and characterization of gelatin-PVP polymer composite scaffold for potential application in bone tissue engineering. Eur. Polym. J. 2019, 119, 155–168. [Google Scholar] [CrossRef]
- Peña-Rodriguez, C.; Martucci, J.F.; Neira, L.M.; Arbelaiz, A.; Eceiza, A.; A Ruseckaite, R. Functional properties and in vitro antioxidant and antibacterial effectiveness of pigskin gelatin films incorporated with hydrolysable chestnut tannin. Food Sci. Technol. Int. 2014, 21, 221–231. [Google Scholar] [CrossRef]
- López-Calderón, H.D.; Avilés-Arnaut, H.; Galán-Wong, L.J.; Almaguer-Cantú, V.; Laguna-Camacho, J.R.; Calderón-Ramón, C.; Escalante-Martínez, J.E.; Arévalo-Niño, K. Electrospun Polyvinylpyrrolidone-Gelatin and Cellulose Acetate Bi-Layer Scaffold Loaded with Gentamicin as Possible Wound Dressing. Polymers 2020, 12, 2311. [Google Scholar] [CrossRef]
- Imtiaz, N.; Khan Niazi, M.B.; Fasim, F.; Ali Khan, B.; Asma Bano, S.; Shah, G.M.; Badshah, M.; Menaa, F.; Uzair, B. Fabrication of an Original Transparent PVA/Gelatin Hydrogel: In Vitro Antimicrobial Activity against Skin Pathogens. Int. J. Polym. Sci. 2019, 2019, 7651810. [Google Scholar] [CrossRef] [Green Version]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. AAPS PharmSciTech 2007, 8, E142–E146. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Polyvinyl Alcohol—Gelatin Patches of Salicylic Acid: Preparation, Characterization and Drug Release Studies. J. Biomater. Appl. 2006, 21, 75–91. [Google Scholar] [CrossRef]
- Guo, X.; Lu, Y.; Cui, H.; Jia, X.; Bai, H.; Ma, Y. Factors Affecting the Physical Properties of Edible Composite Film Prepared from Zein and Wheat Gluten. Molecules 2012, 17, 3794–3804. [Google Scholar] [CrossRef] [Green Version]
- Arvanitoyannis, I.S. Formation and Properties of Collagen and Gelatin films and Coatings. In Protein-Based Films and Coatings, 1st ed.; Gennadios, A., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 275–304. [Google Scholar]
- Thomazine, M.; Carvalho, R.A.; Sobral, P.J. Physical Properties of Gelatin Films Plasticized by Blends of Glycerol and Sorbitol. J. Food Sci. 2006, 70, E172–E176. [Google Scholar] [CrossRef]
- Coppola, M.; Djabourov, M.; Ferrand, M. Phase Diagram of Gelatin Plasticized by Water and Glycerol. Macromol. Symp. 2008, 273, 56–65. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 1–74. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.; Morgenstern, B. Characterization of Gelatine and Acid Soluble Collagen by Size Exclusion Chromatography Coupled with Multi Angle Light Scattering (SEC-MALS). Biomacromolecules 2003, 4, 1727–1732. [Google Scholar] [CrossRef]
- Normand, V.; Muller, S.; Ravey, A.J.-C.; Parker, A. Gelation Kinetics of Gelatin: A Master Curve and Network Modeling. Macromolecules 2000, 33, 1063–1071. [Google Scholar] [CrossRef]
- Rathore, V.S.; Rajawat, J.S.; Chouhan, C.S.; Solanki, N.S. Formulation and Evaluation of Propranolol Hydrochloride Solid Dispersions. Am. J. PharmTech Res. 2013, 3, 302–313. [Google Scholar]
- Ambrus, R.; Gergely, M.; Zvonar, A.; Szabó-Révész, P.; Sipos, E. The Role of co-Spray-Drying Procedure in the Preformulation of Intranasal Propranolol Hydrochloride. Acta Chim. Slov. 2014, 61, 601–607. [Google Scholar] [PubMed]
- Łyszczarz, E.; Brniak, W.; Szafraniec-Szczęsny, J.; Majka, T.M.; Majda, D.; Zych, M.; Pielichowski, K.; Jachowicz, R. The Impact of the Preparation Method on the Properties of Orodispersible Films with Aripiprazole: Electrospinning vs. Casting and 3D PrintingMethods. Pharmaceutics 2021, 13, 1122. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, C.F.; dos Santos, R.S.; da Silva, J.B.; Rosseto, H.C.; Sakita, K.M.; Svidzinski, T.I.E.; Bonfim-Mendonça, P.D.S.; Bruschi, M.L. Development and in vitro evaluation of buccal mucoadhesive films for photodynamic inactivation of Candida albicans. Photodiagnosis Photodyn. Ther. 2020, 32, 101957. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.Y.; Bisson, G.R.; Tsou, A.H. Microindentation on Gelatin Films with a Spherical Indenter—A Viscoelastic Analysis. Mater. Res. Soc. Symp. Proc. 1993, 308, 489–494. [Google Scholar] [CrossRef]
- Mattice, J.M.; Lau, A.; Oyen, M.; Kent, R.W. Spherical indentation load-relaxation of soft biological tissues. J. Mater. Res. 2006, 21, 2003–2010. [Google Scholar] [CrossRef]
- Oyen, M.L. Spherical Indentation Creep Following Ramp Loading. J. Mater. Res. 2005, 20, 2094–2100. [Google Scholar] [CrossRef]
- Lin, D.C.; Shreiber, D.I.; Dimitriadis, E.K.; Horkay, F. Spherical indentation of soft matter beyond the Hertzian regime: Numerical and experimental validation of hyperelastic models. Biomech. Model. Mechanobiol. 2008, 8, 345–358. [Google Scholar] [CrossRef] [Green Version]
- Chua, W.K.; Oyen, M.L. Viscoelastic Properties of Membranes Measured by Spherical Indentation. Cell. Mol. Bioeng. 2009, 2, 49–56. [Google Scholar] [CrossRef]
- Demiröz, F.N.T.; Acartürk, F.; Erdoğan, D. Development of long-acting bioadhesive vaginal gels of oxybutynin: Formulation, in vitro and in vivo evaluations. Int. J. Pharm. 2013, 457, 25–39. [Google Scholar] [CrossRef]
- Kumar, K.; Dhawan, N.; Sharma, H.; Vaidya, S.; Vaidya, B. Bioadhesive polymers: Novel tool for drug delivery. Artif. Cells, Nanomedicine, Biotechnol. 2013, 42, 274–283. [Google Scholar] [CrossRef]
- Çelebioğlu, H.Y.; Lee, S.; Chronakis, I.S. Interactions of salivary mucins and saliva with food proteins: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 64–83. [Google Scholar] [CrossRef] [Green Version]
- Sionkowska, A. Interaction of collagen and poly(vinyl pyrrolidone) in blends. Eur. Polym. J. 2003, 39, 2135–2140. [Google Scholar] [CrossRef]
- Pushp, P.; Bhaskar, R.; Kelkar, S.; Sharma, N.; Pathak, D.; Gupta, M.K. Plasticized poly(vinylalcohol) and poly(vinylpyrrolidone) based patches with tunable mechanical properties for cardiac tissue engineering applications. Biotechnol. Bioeng. 2021, 118, 2312–2325. [Google Scholar] [CrossRef]
- Tagami, T.; Yoshimura, N.; Goto, E.; Noda, T.; Ozeki, T. Fabrication of Muco-Adhesive Oral Films by the 3D Printing of Hydroxypropyl Methylcellulose-Based Catechin-Loaded Formulations. Biol. Pharm. Bull. 2019, 42, 1898–1905. [Google Scholar] [CrossRef] [Green Version]
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef]
- Lopes, C.M.A.; Felisberti, M.I. Mechanical behavior and biocompatibility of poly(1-vinyl-2-pyrrolidinone)-gelatin IPN hydrogels. Biomaterials 2003, 24, 1279–1284. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–84. [Google Scholar]
- Malekjani, N.; Jafari, S.M. Modeling the release of food bioactive ingredients from carriers/nanocarriers by the empirical, semiempirical, and mechanistic models. Compr. Rev. Food Sci. Food Saf. 2020, 20, 3–47. [Google Scholar] [CrossRef]
- Xia, B.; Yang, Z.; Zhou, H.; Lukacova, V.; Zhu, W.; Milewski, M.; Kesisoglou, F. Development of a Novel Oral Cavity Compartmental Absorption and Transit Model for Sublingual Administration: Illustration with Zolpidem. AAPS J. 2015, 17, 631–642. [Google Scholar] [CrossRef] [Green Version]
- Chidsey, C.A.; Morselli, P.; Bianchetti, G.; Morganti, A.; Leonetti, G.; Zanchetti, A. Studies of the absorption and removal of propranolol in hypertensive patients during therapy. Circulation 1975, 52, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Nies, A.S.; Shand, D.G. Clinical pharmacology of propranolol. Circulation 1975, 52, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Mayo Clinic. Drugs and Supplements: Propranolol (Oral Route). Available online: https://www.mayoclinic.org/drugs-supplements/propranolol-oral-route/proper-use/drg-20071164 (accessed on 1 December 2021).
- Medicines. Inderal 10 mg Film-Coated Tablets. Available online: https://www.medicines.org.uk/emc/product/12858/smpc (accessed on 1 December 2021).
- Mansur, A.; Avakian, S.; Paula, R.; Donzella, H.; Santos, S.; Ramires, J.A.F. Pharmacokinetics and pharmacodynamics of propranolol in hypertensive patients after sublingual administration: Systemic availability. Braz. J. Med. Biol. Res. 1998, 31, 691–696. [Google Scholar] [CrossRef] [Green Version]








| Formulations | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Ingredients | ||||||
| Gelatin, type A | 6 g | 6 g | 3 g | 3 g | 3 g | 3 g |
| PVP | 3 g | 3 g | ||||
| PVA | 3 g | 3 g | ||||
| Glycerol 85% | 0.6 g | 0.6 g | 0.6 g | 0.6 g | 0.6 g | 0.6 g |
| PRH | 1.5 g | 1.5 g | 1.5 g | |||
| Purified water | ad 60 g | ad 60 g | ad 60 g | ad 60 g | ad 60 g | ad 60 g |
| OCCAT Parameter | Value |
|---|---|
| Formulation type | Buccal patch a |
| Drug dose | 20 mg a |
| Film surface area | 2.25 cm2 a |
| Oral transit model | Normal swallowing a |
| Saliva production rate (base value) | 0.6 mL/min b |
| Fraction unbound in oral tissue (Fut) | 0.21 c |
| Drug diffusivity through oral mucosa | 7.21 × 10−7 cm2/s c |
| Tensile Strength | Hardness | Strength of Adhesion | ||||
| Factor | F Value | p Value | F Value | p Value | F Value | p Value |
| Polymer | 42.94 | <0.001 | 21.93 | <0.001 | 1.96 | 0.173 |
| Drug | 11.23 | 0.007 | 0.34 | 0.570 | 2.91 | 0.107 |
| Interaction | 0.69 | 0.525 | 0.66 | 0.535 | 1.12 | 0.350 |
| Elastic Modulus | Reduced Modulus | Work of Adhesion | ||||
| Factor | F Value | p Value | F Value | p Value | F Value | p Value |
| Polymer | 3.92 | 0.055 | 12.71 | 0.001 | 4.27 | 0.032 |
| Drug | 0.50 | 0.494 | 0.33 | 0.575 | 8.56 | 0.010 |
| Interaction | 10.79 | 0.003 | 1.89 | 0.194 | 1.78 | 0.201 |
| Formulations | F2 | F4 | F5 |
|---|---|---|---|
| Drug content percentage | 98.27 ± 2.39 | 97.58 ± 3.65 | 95.74 ± 3.17 |
| Model | Zero Order | First Order | Higuchi Model | Korsmeyer–Peppas Model | |||||
|---|---|---|---|---|---|---|---|---|---|
| Criteria | R2 | MSC | R2 | MSC | R2 | MSC | R2 | MSC | n Value |
| Formulation | |||||||||
| F2 | 0.6777 | 1.2998 | 0.9682 | 2.6653 | 0.5167 | 0.0553 | 0.9259 | 2.1537 | 0.215 |
| F4 | 0.8940 | 1.9315 | 0.9902 | 4.3081 | 0.9655 | 3.0539 | 0.9858 | 3.7843 | 0.630 |
| F6 | 0.7281 | 0.9723 | 0.9797 | 3.5694 | 0.9256 | 2.2687 | 0.9268 | 2.7314 | 0.528 |
| Formulation (Dose) | Cmax (ng/mL) | tmax (h) | AUC0–∞ (ng h/mL) | F (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (Range) | CV% | Mean (Range) | CV% | Mean (Range) | CV% | Mean (Range) | CV% | |
| F2 (20 mg) | 42.13 (23.16–71.36) | 26.59 | 3.10 (1.86–4.58) | 19.43 | 404.87 (197.69–803.03) | 34.80 | 99.89 (99.88–99.90) | 2.62 × 10−3 |
| F4 (20 mg) | 40.72 (21.43–68.54) | 24.26 | 4.78 (3.24–7.08) | 16.98 | 451.74 (210.46–808.55) | 31.49 | 99.99 (99.93–100.00) | 1.08 × 10−2 |
| F6 (20 mg) | 41.67 (23.08–70.61) | 26.56 | 3.70 (2.56–5.16) | 15.32 | 404.10 (197.32–801.51) | 34.80 | 99.70 (99.69–99.71) | 2.77 × 10−3 |
| IR tablet (20 mg) | 22.81 (0.74–54.38) | 45.42 | 1.12 (0.65–1.62) | 19.35 | 168.02 (4.44–461.53) | 53.45 | 35.24 (1.00–54.92) | 33.82 |
| IR tablet (40 mg) | 48.14 (1.23–100.97) | 44.76 | 1.08 (0.74–1.62) | 16.99 | 319.63 (5.36–1088.80) | 57.97 | 33.12 (1.32–57.57) | 36.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, M.; Petrović, M.; Cvijić, S.; Tomić, N.; Stojanović, D.; Ibrić, S.; Uskoković, P. 3D Printed Buccal Films for Prolonged-Release of Propranolol Hydrochloride: Development, Characterization and Bioavailability Prediction. Pharmaceutics 2021, 13, 2143. https://doi.org/10.3390/pharmaceutics13122143
Jovanović M, Petrović M, Cvijić S, Tomić N, Stojanović D, Ibrić S, Uskoković P. 3D Printed Buccal Films for Prolonged-Release of Propranolol Hydrochloride: Development, Characterization and Bioavailability Prediction. Pharmaceutics. 2021; 13(12):2143. https://doi.org/10.3390/pharmaceutics13122143
Chicago/Turabian StyleJovanović, Marija, Miloš Petrović, Sandra Cvijić, Nataša Tomić, Dušica Stojanović, Svetlana Ibrić, and Petar Uskoković. 2021. "3D Printed Buccal Films for Prolonged-Release of Propranolol Hydrochloride: Development, Characterization and Bioavailability Prediction" Pharmaceutics 13, no. 12: 2143. https://doi.org/10.3390/pharmaceutics13122143
APA StyleJovanović, M., Petrović, M., Cvijić, S., Tomić, N., Stojanović, D., Ibrić, S., & Uskoković, P. (2021). 3D Printed Buccal Films for Prolonged-Release of Propranolol Hydrochloride: Development, Characterization and Bioavailability Prediction. Pharmaceutics, 13(12), 2143. https://doi.org/10.3390/pharmaceutics13122143