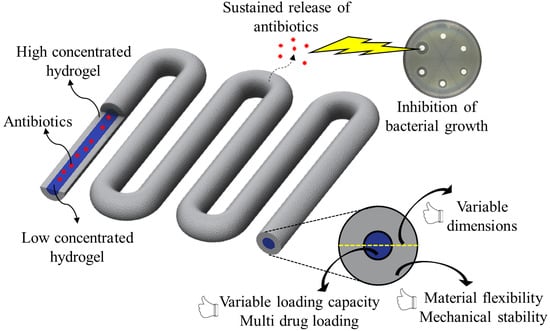
Controlled and Local Delivery of Antibiotics by 3D Core/Shell Printed Hydrogel Scaffolds to Treat Soft Tissue Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Shell Biomaterial Inks
2.2. Preparation of Antibiotics Loaded Core Biomaterial Inks
2.3. Rheological Characterization of the Biomaterial Inks
2.4. Fabrication of Scaffolds by 3D Core/Shell (C/S) Printing
2.5. Release of Antibiotics
2.6. Spectrophotometric Quantification of Antibiotics
2.7. Agar Diffusion Assays to Quantify Antibacterial Activity of the Released Antibiotics
2.8. Statistical Analysis
3. Results
3.1. Fabrication of Antibiotic-Loaded Scaffolds by Core/Shell Extrusion Printing
3.1.1. Antibiotic-Loaded Core Biomaterial Inks
3.1.2. Rheological Characterization of Core and Shell Biomaterial Inks
3.1.3. Extrusion-Printing of Antibiotic-Loaded Core/Shell Scaffolds
3.2. Spectrophotometric Methods for Quantifying Antibiotics in Release Solutions
3.3. Agar Diffusion Assay for Antibiotics Quantification
3.4. Release Kinetics of Antibiotics from C/S Scaffolds
3.4.1. Impact of Shell Composition
3.4.2. Impact of Shell Thickness
4. Discussion
4.1. Loading of Antibiotics in Core Biomaterial Inks
4.2. Establishing Suitable Antibiotic Quantifying Methods
4.3. Release of Antibiotics from Core/Shell Scaffolds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metsemakers, W.J.; Morgenstern, M.; Senneville, E.; Borens, O.; Govaert, G.; Onsea, J.; Depypere, M.; Richards, R.G.; Trampuz, A.; Verhofstad, M.; et al. General treatment principles for fracture-related infection: Recommendations from an international expert group. Arch. Orthop. Trauma Surg. 2020, 140, 1013–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norbury, W.; Herndon, D.N.; Tanksley, J.; Jeschke, M.G.; Finnerty, C.C. Infection in burns. Surg. Infect. 2016, 17, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Prim. 2020, 6, 11. [Google Scholar] [CrossRef]
- Zalavras, C.G. Prevention of Infection in Open Fractures. Infect. Dis. Clin. N. Am. 2017, 31, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Frew, Q.; Green, A.; Martin, R.; Dziewulski, P. Cause of death and correlation with autopsy findings in burns patients. Burns 2013, 39, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Bloemsma, G.C.; Dokter, J.; Boxma, H.; Oen, I.M.M.H. Mortality and causes of death in a burn centre. Burns 2008, 34, 1103–1107. [Google Scholar] [CrossRef]
- Keen, E.F.; Robinson, B.J.; Hospenthal, D.R.; Aldous, W.K.; Wolf, S.E.; Chung, K.K.; Murray, C.K. Incidence and bacteriology of burn infections at a military burn center. Burns 2010, 36, 461–468. [Google Scholar] [CrossRef]
- Oliveira, P.R.; Carvalho, V.C.; da Silva, F.C.; de Paula, A.P.; Santos-Silva, J.; Lima, A.L. The incidence and microbiological profile of surgical site infections following internal fixation of closed and open fractures. Rev. Bras. Ortop. 2016, 51, 396–399. [Google Scholar] [CrossRef] [Green Version]
- Rupp, M.; Popp, D.; Alt, V. Prevention of infection in open fractures: Where are the pendulums now? Injury 2020, 51, S57–S63. [Google Scholar] [CrossRef]
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef] [Green Version]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Ramos, G.; Cornistein, W.; Cerino, G.T.; Nacif, G. Systemic antimicrobial prophylaxis in burn patients: Systematic review. J. Hosp. Infect. 2017, 97, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic delivery strategies to treat skin infections when innate antimicrobial defense fails. Antibiotics 2020, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Working, Z.M.; Frederikse, H.; Drew, A.; Loc-Carrillo, C.; Kubiak, E.N. Bone penetrance of locally administered vancomycin powder in a rat femur fracture model. Injury 2017, 48, 1459–1465. [Google Scholar] [CrossRef]
- Morgenstern, M.; Vallejo, A.; McNally, M.A.; Moriarty, T.F.; Ferguson, J.Y.; Nijs, S.; Metsemakers, W.J. The effect of local antibiotic prophylaxis when treating open limb fractures: A systematic review and meta-analysis. Bone Jt. Res. 2018, 7, 447–456. [Google Scholar] [CrossRef]
- Carver, D.C.; Kuehn, S.B.; Weinlein, J.C. Role of Systemic and Local Antibiotics in the Treatment of Open Fractures. Orthop. Clin. N. Am. 2017, 48, 137–153. [Google Scholar] [CrossRef]
- Sørensen, T.S.; Sørensen, A.I.; Merser, S. Rapid release of gentamicin from collagen sponge. In vitro comparison with plastic beads. Acta Orthop. Scand. 1990, 61, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Grolman, J.M.; Singh, M.; Mooney, D.J.; Eriksson, E.; Nuutila, K. Antibiotic-Containing Agarose Hydrogel for Wound and Burn Care. J. Burn Care Res. 2019, 40, 900–906. [Google Scholar] [CrossRef]
- Nuutila, K.; Grolman, J.; Yang, L.; Broomhead, M.; Lipsitz, S.; Onderdonk, A.; Mooney, D.; Eriksson, E. Immediate Treatment of Burn Wounds with High Concentrations of Topical Antibiotics in an Alginate Hydrogel Using a Platform Wound Device. Adv. Wound Care 2020, 9, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Boo, G.A.T.; Arens, D.; Metsemakers, W.J.; Zeiter, S.; Richards, R.G.; Grijpma, D.W.; Eglin, D.; Moriarty, T.F. Injectable gentamicin-loaded thermo-responsive hyaluronic acid derivative prevents infection in a rabbit model. Acta Biomater. 2016, 43, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Cubo, N.; Garcia, M.; del Cañizo, J.F.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2017, 9, 015006. [Google Scholar] [CrossRef] [Green Version]
- Akkineni, A.R.; Ahlfeld, T.; Lode, A.; Gelinsky, M. A versatile method for combining different biopolymers in a core/shell fashion by 3D plotting to achieve mechanically robust constructs. Biofabrication 2016, 8, 045001. [Google Scholar] [CrossRef]
- Smidsrød, O.; Skjak-Brlk, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Köllmer, M.; Appel, A.A.; Somo, S.I.; Brey, E.M. Long-Term Function of Alginate-Encapsulated Islets. Tissue Eng. Part B 2016, 22, 34–46. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Placht, A.M.; Paul, B.; Brüggemeier, S.; Gelinsky, M.; Lode, A. Three-dimensional plotting of a cell-laden alginate/methylcellulose blend: Towards biofabrication of tissue engineering constructs with clinically relevant dimensions. J. Tissue Eng. Regen. Med. 2017, 11, 1574–1587. [Google Scholar] [CrossRef]
- Hodder, E.; Duin, S.; Kilian, D.; Ahlfeld, T.; Seidel, J.; Nachtigall, C.; Bush, P.; Covill, D.; Gelinsky, M.; Lode, A. Investigating the effect of sterilisation methods on physical properties and cytocompatibility of methyl cellulose used in combination with alginate for 3D-bioplotting of chondrocytes. J. Mater. Sci. Mater. Med. 2019, 30, 10. [Google Scholar] [CrossRef] [PubMed]
- Duin, S.; Schütz, K.; Ahlfeld, T.; Lehmann, S.; Lode, A.; Ludwig, B.; Gelinsky, M. 3D Bioprinting of Functional Islets of Langerhans in an Alginate/Methylcellulose Hydrogel-Blend. Adv. Healthc. Mater. 2019, 8, 1801631. [Google Scholar] [CrossRef] [PubMed]
- Kilian, D.; Ahlfeld, T.; Akkineni, A.R.; Bernhardt, A.; Gelinsky, M.; Lode, A. 3D Bioprinting of osteochondral tissue substitutes —In vitro-chondrogenesis in multi-layered mineralized constructs. Sci. Rep. 2020, 10, 8277. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Cidonio, G.; Kilian, D.; Duin, S.; Akkineni, A.R.; Dawson, J.I.; Yang, S.; Lode, A.; Oreffo, R.O.C.; Gelinsky, M. Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication 2017, 9, 034103. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Cubo-Mateo, N.; Cometta, S.; Guduric, V.; Vater, C.; Bernhardt, A.; Akkineni, A.R.; Lode, A.; Gelinsky, M. A Novel Plasma-based Bioink Stimulates Cell Proliferation and Differentiation in Bioprinted, Mineralized Constructs. ACS Appl. Mater. Interfaces 2020, 12, 12557–12572. [Google Scholar] [CrossRef]
- Guduric, V.; Belton, N.; Richter, R.F.; Bernhardt, A.; Spangenberg, J.; Wu, C.; Lode, A.; Gelinsky, M. Tailorable zinc-substituted mesoporous bioactive glass/alginate-methylcellulose composite bioinks. Materials 2021, 14, 1225. [Google Scholar] [CrossRef] [PubMed]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef] [Green Version]
- Dawson, J.I.; Oreffo, R.O.C. Clay: New Opportunities for Tissue Regeneration and Biomaterial Design. Adv. Mater. 2013, 25, 4069–4086. [Google Scholar] [CrossRef]
- Becher, T.B.; Mendonça, M.C.P.; de Farias, M.A.; Portugal, R.V.; de Jesus, M.B.; Ornelas, C. Soft Nanohydrogels Based on Laponite Nanodiscs: A Versatile Drug Delivery Platform for Theranostics and Drug Cocktails. ACS Appl. Mater. Interfaces 2018, 10, 21891–21900. [Google Scholar] [CrossRef]
- Tomás, H.; Alves, C.S.; Rodrigues, J. Laponite®: A key nanoplatform for biomedical applications? Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2407–2420. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.H.; Mohamed, F.; Rosli, L.M.M.; Shafri, M.A.M.; Haris, M.S.; Adina, A.B. Spectrophotometric determination of gentamicin loaded PLGA microparticles and method validation via Ninhydrin-gentamicin complex as a rapid quantification approach. J. Appl. Pharm. Sci. 2016, 6, 007–014. [Google Scholar] [CrossRef] [Green Version]
- Raja, N.; Yun, H. A simultaneous 3D printing process for the fabrication of bioceramic and cell-laden hydrogel core/shell scaffolds with potential application in bone tissue regeneration. J. Mater. Chem. B 2016, 4, 4707–4716. [Google Scholar] [CrossRef]
- Wei, X.; Liu, C.; Wang, Z.; Luo, Y. 3D printed core-shell hydrogel fiber scaffolds with NIR-triggered drug release for localized therapy of breast cancer. Int. J. Pharm. 2020, 580, 119219. [Google Scholar] [CrossRef] [PubMed]
- Heriot, M.; Nottelet, B.; Garric, X.; D’Este, M.; Richards, G.R.; Moriarty, F.T.; Eglin, D.; Guillaume, O. Interaction of gentamicin sulfate with alginate and consequences on the physico-chemical properties of alginate-containing biofilms. Int. J. Biol. Macromol. 2019, 121, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.A.; Hodges, N.A.; Marriott, C. Antibiotic interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis-derived Pseudomonas aeruginosa. J. Antimicrob. Chemother. 1988, 22, 667–674. [Google Scholar] [CrossRef]
- Becher, T.B.; Braga, C.B.; Bertuzzi, D.L.; Ramos, M.D., Jr.; Hassan, A.; Crespilho, F.N.; Ornelas, C. The structure–property relationship in LAPONITE® materials: From Wigner glasses to strong self-healing hydrogels formed by non-covalent interactions. Soft Matter 2019, 15, 1278–1289. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Monette, C.E.; Bracero, S.; Saha, M.S. Methods for field measurement of antibiotic concentrations: Limitations and outlook. FEMS Microbiol. Ecol. 2018, 94, fiy105. [Google Scholar] [CrossRef]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Bishop, J.R.; Bodine, A.B.; O’Dell, G.D.; Janzen, J.J. Quantitative assay for antibiotics used commonly in treatment of bovine infections. J. Dairy Sci. 1985, 68, 3031–3036. [Google Scholar] [CrossRef]
- Lalpuria, M.; Karwa, V.; Anantheswaran, R.C.; Floros, J.D. Modified agar diffusion bioassay for better quantification of Nisaplin®. J. Appl. Microbiol. 2013, 114, 663–671. [Google Scholar] [CrossRef]
- Choi, Y.S.; Hong, S.R.; Lee, Y.M.; Song, K.W.; Park, M.H.; Nam, Y.S. Study on gelatin-containing artificial skin: I. Preparation and characteristics of novel gelatin-alginate sponge. Biomaterials 1999, 20, 409–417. [Google Scholar] [CrossRef]
- Kostenko, V.; Ceri, H.; Martinuzzi, R.J. Increased tolerance of Staphylococcus aureus to vancomycin in viscous media. FEMS Immunol. Med. Microbiol. 2007, 51, 277–288. [Google Scholar] [CrossRef]
- Henner, J.; Sitrin, R.D. Isoelectric focusing and electrophoretic titration of antibiotics using bioautographic detection. J. Antibiot. 1984, 37, 1475. [Google Scholar] [CrossRef] [Green Version]
- Dawson, J.I.; Kanczler, J.M.; Yang, X.B.; Attard, G.S.; Oreffo, R.O. Clay Gels For the Delivery of Regenerative Microenvironments. Adv. Mater. 2011, 23, 3304–3308. [Google Scholar] [CrossRef]
- Kim, J.O.; Choi, J.Y.; Park, J.K.; Kim, J.H.; Jin, S.G.; Chang, S.W.; Li, D.X.; Hwang, M.R.; Woo, J.S.; Kim, J.A.; et al. Development of clindamycin-loaded wound dressing with polyvinyl alcohol and sodium alginate. Biol. Pharm. Bull. 2008, 31, 2277–2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, V.; Deimling, C.; Morgenstern, M.; D’Este, M.; Puetzler, J.; Zeiter, S.; Arens, D.; Metsemakers, W.J.; Richards, R.G.; Eglin, D.; et al. Local Application of a Gentamicin-Loaded Hydrogel Early After Injury Is Superior to Perioperative Systemic Prophylaxis in a Rabbit Open Fracture Model. J. Orthop. Trauma 2020, 34, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Cahill, S.V.; Kwon, H.K.; Back, J.; Lee, I.; Lee, S.; Alder, K.D.; Hao, Z.; Yu, K.E.; Dussik, C.M.; Kyriakides, T.R.; et al. Locally delivered adjuvant biofilm-penetrating antibiotics rescue impaired endochondral fracture healing caused by MRSA infection. J. Orthop. Res. 2021, 39, 402–414. [Google Scholar] [CrossRef]
- Lin, H.R.; Ou, L.H.; Lin, Y.J.; Ling, M.H. Hollow, pH-sensitive calcium–alginate/poly(acrylic acid) hydrogel beads as drug carriers for vancomycin release. J. Appl. Polym. Sci. 2018, 136, 1878–1886. [Google Scholar] [CrossRef]
- Ratanavaraporn, J.; Chuma, N.; Kanokpanont, S.; Damrongsakkul, S. Beads fabricated from alginate, hyaluronic acid, and gelatin using ionic crosslinking and layer-by-layer coating techniques for controlled release of gentamicin. J. Appl. Polym. Sci. 2018, 136, 46893. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.F.; Ahmed, M.M.; El-Kady, A.M.; El-Hady, B.M.A.; Ibrahim, A.M. Synthesis of Gelatin-Agarose Scaffold for Controlled Antibiotic Delivery and its Modification by Glass Nanoparticles Addition as a Potential Osteomyelitis Treatment. Silicon 2021, 13, 2011–2028. [Google Scholar] [CrossRef]
- Shuklaand, S.; Shukla, A. Tunable antibiotic delivery from gellan hydrogels. J. Mater. Chem. B 2018, 6, 6444–6458. [Google Scholar] [CrossRef]
- Kumari, S.; Bargel, H.; Scheibel, T. Recombinant Spider Silk–Silica Hybrid Scaffolds with Drug-Releasing Properties for Tissue Engineering Applications. Macromol. Rapid Commun. 2020, 41, 1900426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldrich, A.; Kuss, M.A.; Duan, B.; Kielian, T. 3D Bioprinted Scaffolds Containing Viable Macrophages and Antibiotics Promote Clearance of Staphylococcus aureus Craniotomy-Associated Biofilm Infection. ACS Appl. Mater. Interfaces 2019, 11, 12298–12307. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, T.A.G.; Arts, J.J.; Geurts, J.A.P. Antibiotic-Loaded Polymethylmethacrylate Beads and Spacers in Treatment of Orthopedic Infections and the Role of Biofilm Formation. Front. Microbiol. 2019, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Seol, Y.J.; Ko, I.K.; Kang, H.W.; Lee, Y.K.; Yoo, J.J.; Atala, A.; Lee, S.J. 3D Bioprinted Human Skeletal Muscle Constructs for Muscle Function Restoration. Sci. Rep. 2018, 8, 12307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, P.R.; Murray, E.; McAdam, C.J.; McConnell, M.A.; Cabral, J.D. Peptide Chitosan/Dextran Core/Shell Vascularized 3D Constructs for Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 32328–32339. [Google Scholar] [CrossRef]







| Properties | Vancomycin | Clindamycin | Gentamicin |
|---|---|---|---|
| Molecular weight (g/moL) | 1485.7 | 461.4 | 575.67 |
| Isoelectric point (pI) | 8.1 [51] | NA | 9.5 [51] |
| pKa (strongest acidic) * | 2.99 | 12.16 | 12.55 |
| pKa (strongest basic) * | 9.93 | 7.55 | 10.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkineni, A.R.; Spangenberg, J.; Geissler, M.; Reichelt, S.; Buechner, H.; Lode, A.; Gelinsky, M. Controlled and Local Delivery of Antibiotics by 3D Core/Shell Printed Hydrogel Scaffolds to Treat Soft Tissue Infections. Pharmaceutics 2021, 13, 2151. https://doi.org/10.3390/pharmaceutics13122151
Akkineni AR, Spangenberg J, Geissler M, Reichelt S, Buechner H, Lode A, Gelinsky M. Controlled and Local Delivery of Antibiotics by 3D Core/Shell Printed Hydrogel Scaffolds to Treat Soft Tissue Infections. Pharmaceutics. 2021; 13(12):2151. https://doi.org/10.3390/pharmaceutics13122151
Chicago/Turabian StyleAkkineni, Ashwini Rahul, Janina Spangenberg, Michael Geissler, Saskia Reichelt, Hubert Buechner, Anja Lode, and Michael Gelinsky. 2021. "Controlled and Local Delivery of Antibiotics by 3D Core/Shell Printed Hydrogel Scaffolds to Treat Soft Tissue Infections" Pharmaceutics 13, no. 12: 2151. https://doi.org/10.3390/pharmaceutics13122151
APA StyleAkkineni, A. R., Spangenberg, J., Geissler, M., Reichelt, S., Buechner, H., Lode, A., & Gelinsky, M. (2021). Controlled and Local Delivery of Antibiotics by 3D Core/Shell Printed Hydrogel Scaffolds to Treat Soft Tissue Infections. Pharmaceutics, 13(12), 2151. https://doi.org/10.3390/pharmaceutics13122151