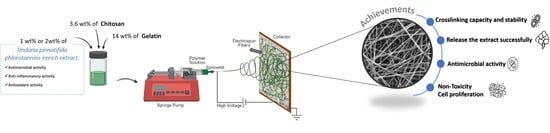
Multifunctional Gelatin/Chitosan Electrospun Wound Dressing Dopped with Undaria pinnatifida Phlorotannin-Enriched Extract for Skin Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Phlorotannin-Enriched Extract Preparation
2.3. Electrospun Meshes Preparation
2.4. Physicochemical Characterization
2.4.1. Apparent Density and Porosity
2.4.2. Morphology and Fiber Diameter
2.4.3. Chemical Characterization
2.4.4. Dissolvability and Water Uptake
2.4.5. Water Vapor Permeability
2.4.6. Contact Angle
2.5. Mechanical Properties
2.6. Hydrolytic and Enzymatic Degradation
2.7. Extract Delivery
2.8. Antimicrobial Activity by Disc Diffusion Assay
2.9. In Vitro Studies
2.9.1. Cytotoxicity
2.9.2. Proliferation Assays
2.10. Statistical Analysis
3. Results and Discussion
3.1. Morphology and Fiber Diameter
3.2. Physiochemical and Structural Characterization
3.3. Water Uptake, Dissolvability, Water Permeability, and Contact Angle
3.4. Mechanical Properties
3.5. Hydrolytic and Enzymatic Degradation
3.6. In Vitro Phlorotannins-Enriched Extract Release
3.7. Antimicrobial Activity
3.8. Biological Behavior
3.8.1. Cytotoxicity
3.8.2. Cell Metabolic Activity and Proliferation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.; Singh, S. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Advancement of wound care from grafts to bioengineered smart skin substitutes. Prog. Biomater. 2014, 3, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Kim, G. Electrospun PCL/phlorotannin nanofibres for tissue engineering: Physical properties and cellular activities. Carbohydr. Polym. 2012, 90, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Halstead, F.D.; Rauf, M.; Bamford, A.; Wearn, C.M.; Bishop, J.R.B.; Burt, R.; Fraise, A.P.; Moiemen, N.S.; Oppenheim, B.A.; Webber, M.A. Antimicrobial dressings: Comparison of the ability of a panel of dressings to prevent biofilm formation by key burn wound pathogens. Burns 2015, 41, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human wounds and its burden: An updated compendium of estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Posnett, J.; Gottrup, F.; Lundgren, H.; Saal, G. The resource impact of wounds on health-care providers in Europe. J. Wound Care 2009, 18, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Wound Care Market—Global Forecast to 2026|MarketsandMarkets. Available online: https://www.marketsandmarkets.com/Market-Reports/wound-care-market-371.html (accessed on 29 April 2021).
- Dias, J.R.; Baptista-Silva, S.; De Oliveira, C.M.T.; Sousa, A.; Oliveira, A.L.; Bártolo, P.J.; Granja, P.L. In Situ crosslinked electrospun gelatin nanofibers for skin regeneration. Eur. Polym. J. 2017, 95, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.R.; dos Santos, C.; Horta, J.; Granja, P.L.; Bártolo, P.J. A new design of an electrospinning apparatus for tissue engineering applications. Int. J. Bioprinting 2017, 3, 002. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hu, S.; Jia, Q.; Zhang, L. Advances in electrospinning of natural biomaterials for wound dressing. J. Nanomater. 2020, 2020, 8719859. [Google Scholar] [CrossRef] [Green Version]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun polymeric nanofibres as wound dressings: A review. Colloids Surf. B Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef]
- Khajavi, R.; Abbasipour, M. Controlling Nanofiber Morphology by the Electrospinning Process, 1st ed.; Afshari, M., Ed.; Woodhead Publishing: Cambridge, UK, 2017; ISBN 9780081005514. [Google Scholar]
- Balusamy, B.; Senthamizhan, A.; Uyar, T. Electrospun nanofibrous materials for wound healing applications. In Electrospun Materials for Tissue Engineering and Biomedical Applications: Research, Design and Commercialization; Kny, E., Uyar, T., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 147–177. ISBN 9780081022221. [Google Scholar]
- Willerth, S.M. Electrospun nanofibers for diverse applications. In Comprehensive Nanoscience and Nanotechnology; Andrews, D.L., Lipson, R.H., Nann, T., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 275–286. ISBN 9780128035818. [Google Scholar]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Dias, J.R.; Granja, P.L.; Bártolo, P.J. Advances in electrospun skin substitutes. Prog. Mater. Sci. 2016, 84, 314–334. [Google Scholar] [CrossRef]
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun nanofibers for customized drug-delivery systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Samimi Gharaie, S.; Habibi, S.; Nazockdast, H. Fabrication and characterization of chitosan/gelatin/thermoplastic polyurethane blend nanofibers. J. Text. Fibrous Mater. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Jafari, J.; Emami, S.H.; Samadikuchaksaraei, A.; Bahar, M.A.; Gorjipour, F. Electrospun chitosan-gelatin nanofiberous scaffold: Fabrication and in vitro evaluation. Biomed. Mater. Eng. 2011, 21, 99–112. [Google Scholar] [CrossRef]
- González de Torre, I.; Ibáñez-Fonseca, A.; Quintanilla, L.; Alonso, M.; Rodríguez-Cabello, J.C. Random and oriented electrospun fibers based on a multicomponent, in situ clickable elastin-like recombinamer system for dermal tissue engineering. Acta Biomater. 2018, 72, 137–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, B.; Wang, T.; Li, Z.; Dai, F.; Lv, L.; Tang, F.; Yu, K.; Liu, J.; Lan, G. Healing of skin wounds with a chitosan-gelatin sponge loaded with tannins and platelet-rich plasma. Int. J. Biol. Macromol. 2016, 82, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Talebian, A.; Mansourian, A. Release of Vancomycin from electrospun gelatin/chitosan nanofibers. Mater. Today Proc. 2017, 4, 7065–7069. [Google Scholar] [CrossRef]
- Kenawy, E.; Abdel-Hay, F.I.; El-Newehy, M.H.; Wnek, G.E. Processing of Polymer Nanofibers through Electrospinning as Drug Delivery Systems. In Nanomaterials: Risks and Benefits; Linkov, I., Steevens, J., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 247–263. [Google Scholar]
- Jalaja, K.; Naskar, D.; Kundu, S.C.; James, N.R. Potential of electrospun core-shell structured gelatin-chitosan nanofibers for biomedical applications. Carbohydr. Polym. 2016, 136, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Ige, O.O.; Umoru, L.E.; Aribo, S. Natural Products: A minefield of biomaterials. Int. Sch. Res. Netw. 2012, 2012, 983062. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.A.M.; Félix, R.; Félix, C.; Januário, A.P.; Alves, N.; Novais, S.C.; Dias, J.R.; Lemos, M.F.L. A Biorefinery approach to the biomass of the seaweed Undaria pinnatifida (Harvey Suringar, 1873): Obtaining phlorotannins-enriched extracts for wound healing. Biomolecules 2021, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; De Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti. Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.A.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from brown seaweeds as a potential antimicrobial agent in animal feeds. ACS Omega 2020, 5, 9093–9103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurahashi, T.; Fujii, J. Roles of antioxidative enzymes in wound healing. J. Dev. Biol. 2015, 3, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Félix, R.; Valentão, P.; Andrade, P.B.; Félix, C.; Novais, S.C.; Lemos, M.F.L. Evaluating the in vitro potential of natural extracts to protect lipids from oxidative damage. Antioxidants 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shavandi, A.; Bekhit, A.E.D.A.; Saeedi, P.; Izadifar, Z.; Bekhit, A.A.; Khademhosseini, A. Polyphenol uses in biomaterials engineering. Biomaterials 2018, 167, 91–106. [Google Scholar] [CrossRef]
- Gao, X.; Xu, Z.; Liu, G.; Wu, J. Polyphenols as a versatile component in tissue engineering. Acta Biomater. 2021, 119, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, I.; Baptista-Silva, S.; Pintado, M.; Oliveira, A. Polyphenols: A promising avenue in therapeutic solutions for wound care. Appl. Sci. 2021, 11, 1230. [Google Scholar] [CrossRef]
- Yeo, M.; Jung, W.-K.; Kim, G. Fabrication, characterisation and biological activity of phlorotannin-conjugated PCL/β-TCP composite scaffolds for bone tissue regeneration. J. Mater. Chem. 2012, 22, 3568–3577. [Google Scholar] [CrossRef]
- Park, H.-H.; Ko, S.-C.; Oh, G.-W.; Heo, S.-J.; Kang, D.-H.; Bae, S.-Y.; Jung, W.-K. Fabrication and characterization of phlorotannins/poly (vinyl alcohol) hydrogel for wound healing application. J. Biomater. Sci. Polym. Ed. 2018, 29, 972–983. [Google Scholar] [CrossRef]
- Kuntzler, S.G.; Costa, J.A.V.; de Morais, M.G. Development of electrospun nanofibers containing chitosan/PEO blend and phenolic compounds with antibacterial activity. Int. J. Biol. Macromol. 2018, 117, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki-Modaress, M.; Zandi, M.; Rajabi, S. Tailoring the gelatin/chitosan electrospun scaffold for application in skin tissue engineering: An in vitro study. Prog. Biomater. 2018, 7, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Mohammadzadehmoghadam, S.; Dong, Y. Fabrication and characterization of electrospun silk fibroin/gelatin scaffolds crosslinked with glutaraldehyde vapor. Front. Mater. 2019, 6, 91. [Google Scholar] [CrossRef]
- The American Society for Testing and Materials. Annual Book of ASTM Standards: ASTM E96-95: Standard Test Methods for Water Vapor Transmission of Materials; American Society for Testing and Materials: Philadelphia, PA, USA, 1995; Volume 552, pp. 785–792. [Google Scholar]
- Tallian, C.; Tegl, G.; Quadlbauer, L.; Vielnascher, R.; Weinberger, S.; Cremers, R.; Pellis, A.; Salari, J.W.O.; Guebitz, G.M. Lysozyme-responsive spray-dried chitosan particles for early detection of wound infection. ACS Appl. Bio Mater. 2019, 2, 1331–1339. [Google Scholar] [CrossRef]
- Lončarević, A.; Ivanković, M.; Rogina, A. Lysozyme-induced degradation of chitosan: The characterisation of degraded chitosan scaffolds. J. Tissue Repair Regen. 2017, 1, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Mak, Y.W.; Leung, W.W.F. Crosslinking of genipin and autoclaving in chitosan-based nanofibrous scaffolds: Structural and physiochemical properties. J. Mater. Sci. 2019, 54, 10941–10962. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, K.H.; Dighe, P.; Kharat, A.R.; Patil, S.V. Mathematical Models of Drug Dissolution: A Review. Sch. Acad. J. Pharm. 2014, 3, 2320–4206. [Google Scholar]
- Kalani, M.M.; Nourmohammadi, J.; Negahdari, B.; Rahimi, A.; Sell, S.A. Electrospun core-sheath poly(vinyl alcohol)/silk fibroin nanofibers with Rosuvastatin release functionality for enhancing osteogenesis of human adipose-derived stem cells. Mater. Sci. Eng. C 2019, 99, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). CLSI Supplement M100S: Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; Clinical and Laboratory Standards Institute: Philadelphia, PA, USA, 2014. [Google Scholar]
- Martí, M.; Frígols, B.; Serrano-Aroca, A. Antimicrobial characterization of advanced materials for bioengineering applications. J. Vis. Exp. 2018, 138, 57710. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization. “UNI EN ISO 10993-5: 2009” Biological Evaluation of Medical Devices–Part 5: In Vitro Cytotoxicity Testing; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Dias, J.R.; Baptista-Silva, S.; Sousa, A.; Oliveira, A.L.; Bártolo, P.J.; Granja, P.L. Biomechanical performance of hybrid electrospun structures for skin regeneration. Mater. Sci. Eng. C 2018, 93, 816–827. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Caramella, C.; Ferrari, F. Electrospinning Technologies in Wound Dressing Applications. In Therapeutic Dressings and Wound Healing Applications; Boateng, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 315–336. [Google Scholar]
- Goh, Y.F.; Shakir, I.; Hussain, R. Electrospun fibers for tissue engineering, drug delivery, and wound dressing. J. Mater. Sci. 2013, 48, 3027–3054. [Google Scholar] [CrossRef]
- Farshi Azhar, F.; Olad, A.; Salehi, R. Fabrication and characterization of chitosan–gelatin/nanohydroxyapatite–polyaniline composite with potential application in tissue engineering scaffolds. Des. Monomers Polym. 2014, 17, 654–667. [Google Scholar] [CrossRef]
- Jridi, M.; Hajji, S.; Ayed, H.B.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, structural, antioxidant and antimicrobial properties of gelatin–chitosan composite edible films. Int. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef]
- Nieto-Suárez, M.; López-Quintela, M.A.; Lazzari, M. Preparation and characterization of crosslinked chitosan/gelatin scaffolds by ice segregation induced self-assembly. Carbohydr. Polym. 2016, 141, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Martucci, J.F.; Espinosa, J.P.; Ruseckaite, R.A. Physicochemical properties of films based on bovine gelatin cross-linked with 1,4-Butanediol Diglycidyl Ether. Food Bioprocess Technol. 2015, 8, 1645–1656. [Google Scholar] [CrossRef]
- Amiri, N.; Rozbeh, Z.; Afrough, T.; Sajadi Tabassi, S.A.; Moradi, A.; Movaffagh, J. Optimization of chitosan-gelatin nanofibers production: Investigating the effect of solution properties and working parameters on fibers diameter. Bionanoscience 2018, 8, 778–789. [Google Scholar] [CrossRef]
- Noorani, B.; Tabandeh, F.; Yazdian, F.; Soheili, Z.-S.; Shakibaie, M.; Rahmani, S. Thin natural gelatin/chitosan nanofibrous scaffolds for retinal pigment epithelium cells. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 754–763. [Google Scholar] [CrossRef]
- Bazmandeh, A.Z.; Mirzaei, E.; Fadaie, M.; Shirian, S.; Ghasemi, Y. Dual spinneret electrospun nanofibrous/gel structure of chitosan-gelatin/chitosan-hyaluronic acid as a wound dressing: in vitro and in vivo studies. Int. J. Biol. Macromol. 2020, 162, 359–373. [Google Scholar] [CrossRef]
- Naseri, N.; Algan, C.; Jacobs, V.; John, M.; Oksman, K.; Mathew, A.P. Electrospun chitosan-based nanocomposite mats reinforced with chitin nanocrystals for wound dressing. Carbohydr. Polym. 2014, 109, 7–15. [Google Scholar] [CrossRef]
- Letha, S.S.; Kumar, A.S.; Nisha, U.; Rosemary, M.J. Electrospun polyurethane-gelatin artificial skin scaffold for wound healing. J. Text. Inst. 2021, 1–10. [Google Scholar] [CrossRef]
- Roy, S.; Zhai, L.; Chan Kim, H.; Hoa Pham, D.; Alrobei, H.; Kim, J. Tannic-acid-cross-linked and TiO 2-nanoparticle reinforced chitosan-based nanocomposite film. Polymers 2021, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.Y.; Wang, Z.M.; Ren, J.; Zhang, C.Y. Electrospinning of gelatin and gelatin/poly(l-lactide) blend and its characteristics for wound dressing. Mater. Sci. Eng. C 2009, 29, 1822–1828. [Google Scholar] [CrossRef]
- Franco, R.A.; Nguyen, T.H.; Lee, B.-T. Preparation and characterization of electrospun PCL/PLGA membranes and chitosan/gelatin hydrogels for skin bioengineering applications. J. Mater. Sci. Mater. Med. 2011, 22, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Lim, S.; Kim, E.; Jeon, I.O.; Choi, Y.S. Preparation of in situ injectable chitosan/gelatin hydrogel using an acid-tolerant tyrosinase. Biotechnol. Bioprocess Eng. 2018, 23, 500–506. [Google Scholar] [CrossRef]
- Morgado, P.I.; Aguiar-Ricardo, A.; Correia, I.J. Asymmetric membranes as ideal wound dressings: An overview on production methods, structure, properties and performance relationship. J. Memb. Sci. 2015, 490, 139–151. [Google Scholar] [CrossRef]
- Wang, J.C. Young’s modulus of porous materials—Part 1 Theoretical derivation of modulus-porosity correlation. J. Mater. Sci. 1984, 19, 801–808. [Google Scholar] [CrossRef]
- Rocasalbas, G.; Francesko, A.; Touriño, S.; Fernández-Francos, X.; Guebitz, G.M.; Tzanov, T. Laccase-assisted formation of bioactive chitosan/gelatin hydrogel stabilized with plant polyphenols. Carbohydr. Polym. 2013, 92, 989–996. [Google Scholar] [CrossRef]
- Yang, C.; Xu, L.; Zhou, Y.; Zhang, X.; Huang, X.; Wang, M.; Han, Y.; Zhai, M.; Wei, S.; Li, J. A green fabrication approach of gelatin/CM-chitosan hybrid hydrogel for wound healing. Carbohydr. Polym. 2010, 82, 1297–1305. [Google Scholar] [CrossRef]
- Zheng, J.P.; Wang, C.Z.; Wang, X.X.; Wang, H.Y.; Zhuang, H.; De Yao, K. Preparation of biomimetic three-dimensional gelatin/montmorillonite–chitosan scaffold for tissue engineering. React. Funct. Polym. 2007, 67, 780–788. [Google Scholar] [CrossRef]
- Kellogg, J.; Grace, M.H.; Lila, M.A. Phlorotannins from alaskan seaweed inhibit carbolytic enzyme activity. Mar. Drugs 2014, 12, 5277–5294. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Hezaveh, H.; Muhamad, I.I. Controlled drug release via minimization of burst release in pH-response kappa-carrageenan/polyvinyl alcohol hydrogels. Chem. Eng. Res. Des. 2013, 91, 508–519. [Google Scholar] [CrossRef]
- Karuppuswamy, P.; Reddy Venugopal, J.; Navaneethan, B.; Luwang Laiva, A.; Ramakrishna, S. Polycaprolactone nanofibers for the controlled release of tetracycline hydrochloride. Mater. Lett. 2015, 141, 180–186. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Vargas, M.; Chiralt, A.; González-Martínez, C. Release of polyphenols from starch-chitosan based films containing thyme extract. Carbohydr. Polym. 2017, 175, 122–130. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag. Shelf Life 2018, 18, 42–50. [Google Scholar] [CrossRef]
- Shao, S.; Li, L.; Yang, G.; Li, J.; Luo, C.; Gong, T.; Zhou, S. Controlled green tea polyphenols release from electrospun PCL/MWCNTs composite nanofibers. Int. J. Pharm. 2011, 421, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Helary, C.; Abed, A.; Mosser, G.; Louedec, L.; Letourneur, D.; Coradin, T.; Giraud-Guille, M.M.; Meddahi-Pellé, A. Evaluation of dense collagen matrices as medicated wound dressing for the treatment of cutaneous chronic wounds. Biomater. Sci. 2015, 3, 373–382. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- Arkoun, M.; Daigle, F.; Heuzey, M.C.; Ajji, A. Mechanism of action of electrospun chitosan-based nanofibers against meat spoilage and pathogenic bacteria. Molecules 2017, 22, 585. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Nada, A.A.; El Aref, A.T.; Sharaf, S.S. The synthesis and characterization of zinc-containing electrospun chitosan/gelatin derivatives with antibacterial properties. Int. J. Biol. Macromol. 2019, 133, 538–544. [Google Scholar] [CrossRef]
- Surendhiran, D.; Cui, H.; Lin, L. Encapsulation of phlorotannin in Alginate/PEO blended nanofibers to preserve chicken meat from Salmonella contaminations. Food Packag. Shelf Life 2019, 100346. [Google Scholar] [CrossRef]
- Eom, S.-H.; Kim, Y.-M.; Kim, S.-K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef] [PubMed]
- Knoll, K.E.; Lindeque, Z.; Adeniji, A.A.; Oosthuizen, C.B.; Lall, N.; Loots, D.T. Elucidating the antimycobacterial mechanism of action of ciprofloxacin using metabolomics. Microorganisms 2021, 9, 1158. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem. 2016, 194, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jeong, M.; Ko, S.; Heo, S.; Kang, H.W.; Kim, S.W.; Hwang, C.W.; Lee, K.D.; Oak, C.; Jung, M.J.; et al. Fabrication and biological activity of polycaprolactone/phlorotannin endotracheal tube to prevent tracheal stenosis: An in vitro and in vivo study. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2020, 108, 1046–1056. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, H.S.; Oh, S.J.; Hwang, C.W.; Jung, W.K. Phlorotannins ameliorate extracellular matrix production in human vocal fold fibroblasts and prevent vocal fold fibrosis via aerosol inhalation in a laser-induced fibrosis model. J. Tissue Eng. Regen. Med. 2020, 14, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Sisson, K.; Zhang, C.; Farach-Carson, M.C.; Chase, D.B.; Rabolt, J.F. Fiber diameters control osteoblastic cell migration and differentiation in electrospun gelatin. J. Biomed. Mater. Res. Part. A 2010, 94, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Ameer, J.M.; PR, A.K.; Kasoju, N. Strategies to tune electrospun scaffold porosity for effective cell response in tissue engineering. J. Funct. Biomater. 2019, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Sun, W.; Kim, J.P.; Lu, X.; Li, Q.; Lin, M.; Mrowczynski, O.; Rizk, E.B.; Cheng, J.; Qian, G.; et al. Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. 2018, 72, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Van Ta, Q.; Mendis, E.; Rajapakse, N.; Jung, W.K.; Byun, H.G.; Jeon, Y.J.; Kim, S.K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kim, H.S.; Oh, J.Y.; Je, J.G.; Jeon, Y.J.; Ryu, B.M. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against UVB-induced damage in vitro in human dermal fibroblasts and in vivo in zebrafish. Food Chem. Toxicol. 2020, 136, 110963. [Google Scholar] [CrossRef] [PubMed]





| \ | Apparent Density (g·cm−3) | Porosity (%) | Average Fiber Diameter (nm) | Swelling Degree (%) | Dissolvability (%) | WVP (g·m−2·day−1) | Young’s Modulus (MPa) | Tensile Strength at Break (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|---|---|---|
| WOE | 0.30 ± 0.09 | 88. 63 ± 1.81 | 388 ± 82 | 405.33 ± 19.11 | 9.92 ± 0.67 | 1207.06 ± 14.97 | 0.174 ± 0.103 | 0.15 ± 0.06 | 9.30 ± 44 |
| WE1 | 0.25 ± 0.03 | 89.22 ± 1.15 b | 302 ± 83 a,b | 458.03 ± 41.52 a,b | 16.35 ± 0.52 a,b | 1220.76 ± 12.06 | 0.055 ± 0.017 a | 0.13 ± 0.08 | 13.59 ± 3.99 a |
| WE2 | 0.31 ± 0.09 | 85.93 ± 1. 58 a | 229 ± 43 a | 516.90 ± 39.75 a | 9.05 ± 0.69 | 1201.056 ± 6.00 | 0.063 ± 0.019 a | 0.08 ± 0.04 a | 15.37 ± 2.63 a |
| Sample | n | K | R2 |
|---|---|---|---|
| WE1 | 0.25 ± 0.009 | 13.03 ± 0.87 | 0.98 |
| WE2 | 0.35 ± 0.0095 | 14.69 ± 0.98 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, C.A.M.; Januário, A.P.; Félix, R.; Alves, N.; Lemos, M.F.L.; Dias, J.R. Multifunctional Gelatin/Chitosan Electrospun Wound Dressing Dopped with Undaria pinnatifida Phlorotannin-Enriched Extract for Skin Regeneration. Pharmaceutics 2021, 13, 2152. https://doi.org/10.3390/pharmaceutics13122152
Ferreira CAM, Januário AP, Félix R, Alves N, Lemos MFL, Dias JR. Multifunctional Gelatin/Chitosan Electrospun Wound Dressing Dopped with Undaria pinnatifida Phlorotannin-Enriched Extract for Skin Regeneration. Pharmaceutics. 2021; 13(12):2152. https://doi.org/10.3390/pharmaceutics13122152
Chicago/Turabian StyleFerreira, Carolina A. M., Adriana P. Januário, Rafael Félix, Nuno Alves, Marco F. L. Lemos, and Juliana R. Dias. 2021. "Multifunctional Gelatin/Chitosan Electrospun Wound Dressing Dopped with Undaria pinnatifida Phlorotannin-Enriched Extract for Skin Regeneration" Pharmaceutics 13, no. 12: 2152. https://doi.org/10.3390/pharmaceutics13122152
APA StyleFerreira, C. A. M., Januário, A. P., Félix, R., Alves, N., Lemos, M. F. L., & Dias, J. R. (2021). Multifunctional Gelatin/Chitosan Electrospun Wound Dressing Dopped with Undaria pinnatifida Phlorotannin-Enriched Extract for Skin Regeneration. Pharmaceutics, 13(12), 2152. https://doi.org/10.3390/pharmaceutics13122152