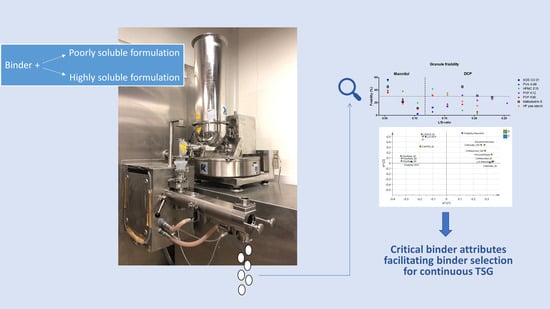
Identifying Critical Binder Attributes to Facilitate Binder Selection for Efficient Formulation Development in a Continuous Twin Screw Wet Granulation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Granules
2.2.2. Granule Evaluation
2.2.3. Preparation of Tablets
2.2.4. Tablet Evaluation
2.2.5. Binder Characterization
2.2.6. Multivariate Data Analysis
3. Results and Discussion
3.1. Mannitol Formulation
3.1.1. Granulation Experiments
3.1.2. Binder Characterization
3.1.3. Correlation between Binder Attributes and Binder Effectiveness
3.1.4. Tablet Characterization
3.2. Binder Selection: DCP versus Mannitol Formulation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vervaet, C.; Remon, J.P. Continuous granulation in the pharmaceutical industry. Chem. Eng. Sci. 2005, 60, 3949–3957. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Vervaet, C. Recent progress in continuous manufacturing of oral solid dosage forms. Int. J. Pharm. 2020, 579, 119194. [Google Scholar] [CrossRef] [PubMed]
- Teżyk, M.; Milanowski, B.; Ernst, A.; Lulek, J. Recent progress in continuous and semi-continuous processing of solid oral dosage forms: A review. Drug Dev. Ind. Pharm. 2015, 42, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Plumb, K. Continuous processing in the pharmaceutical industry: Changing the mind set. Chem. Eng. Res. Des. 2005, 83, 730–738. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration, Guidance for Industry PAT: A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance 2004. Available online: https://www.fda.gov/media/71012/download (accessed on 1 November 2020).
- Lee, S.L. Quality considerations for continuous manufacturing: Guidance for industry. 2019. Available online: https://www.fda.gov/media/121314/download (accessed on 1 December 2020).
- De Leersnyder, F.; Vanhoorne, V.; Bekaert, H.; Vercruysse, J.; Ghijs, M.; Bostijn, N.; Verstraeten, M.; Cappuyns, P.; Van Assche, I.; Vander Heyden, Y.; et al. Breakage and drying behaviour of granules in a continuous fluid bed dryer: Influence of process parameters and wet granule transfer. Eur. J. Pharm. Sci. 2018, 115, 223–232. [Google Scholar] [CrossRef]
- Keleb, E.I.; Vermeire, A.; Vervaet, C.; Remon, J.P. Twin screw granulation as a simple and efficient tool for continuous wet granulation. Int. J. Pharm. 2004, 273, 183–194. [Google Scholar] [CrossRef]
- El Hagrasy, A.S.; Hennenkamp, J.R.; Burke, M.D.; Cartwright, J.J.; Litster, J.D. Twin screw wet granulation: Influence of formulation parameters on granule properties and growth behavior. Powder Technol. 2013, 238, 108–115. [Google Scholar] [CrossRef]
- Vercruysse, J.; Córdoba Díaz, D.; Peeters, E.; Fonteyne, M.; Delaet, U.; Van Assche, I.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous twin screw granulation: Influence of process variables on granule and tablet quality. Eur. J. Pharm. Biopharm. 2012, 82, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Portier, C.; Pandelaere, K.; Delaet, U.; Vigh, T.; Di Pretoro, G.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Continuous twin screw granulation: A complex interplay between formulation properties, process settings and screw design. Int. J. Pharm. 2020, 576, 119004. [Google Scholar] [CrossRef]
- Meier, R.; Moll, K.P.; Krumme, M.; Kleinebudde, P. Impact of fill-level in twin-screw granulation on critical quality attributes of granules and tablets. Eur. J. Pharm. Biopharm. 2017, 115, 102–112. [Google Scholar] [CrossRef]
- Liu, H.; Galbraith, S.C.; Ricart, B.; Stanton, C.; Smith-Goettler, B.; Verdi, L.; O’Connor, T.; Lee, S.; Yoon, S. Optimization of critical quality attributes in continuous twin-screw wet granulation via design space validated with pilot scale experimental data. Int. J. Pharm. 2017, 525, 249–263. [Google Scholar] [CrossRef]
- Willecke, N.; Vervaet, C.; De Beer, T.; Szepes, A.; Wunderlich, M. Formulation design for continuous twin screw wet granulation. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2017. [Google Scholar]
- Yu, S.; Reynolds, G.K.; Huang, Z.; De Matas, M.; Salman, A.D. Granulation of increasingly hydrophobic formulations using a twin screw granulator. Int. J. Pharm. 2014, 475, 82–96. [Google Scholar] [CrossRef]
- Vandevivere, L.; Denduyver, P.; Portier, C.; Häusler, O.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Influence of binder attributes on binder effectiveness in a continuous twin screw wet granulation process via wet and dry binder addition. Int. J. Pharm. 2020, 585, 119466. [Google Scholar] [CrossRef]
- Portier, C.; Pandelaere, K.; Delaet, U.; Vigh, T.; Kumar, A.; Di Pretoro, G.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Continuous twin screw granulation: Influence of process and formulation variables on granule quality attributes of model formulations. Int. J. Pharm. 2020, 576, 118981. [Google Scholar] [CrossRef]
- Bansal, A.K.; Balwani, G.; Sheokand, S. Critical Material Attributes in Wet Granulation; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128104606. [Google Scholar]
- Zarmpi, P.; Flanagan, T.; Meehan, E.; Mann, J.; Østergaard, J.; Fotaki, N. Biopharmaceutical implications of excipient variability on drug dissolution from immediate release products. Eur. J. Pharm. Biopharm. 2020, 154, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Willecke, N.; Szepes, A.; Wunderlich, M.; Remon, J.P.; Vervaet, C.; De Beer, T. Identifying overarching excipient properties towards an in-depth understanding of process and product performance for continuous twin-screw wet granulation. Int. J. Pharm. 2017, 522, 234–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhenge, R.M.; Cartwright, J.J.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Effects of properties of granulation liquid. Powder Technol. 2012, 229, 126–136. [Google Scholar] [CrossRef]
- Chan Seem, T.; Rowson, N.A.; Gabbott, I.; de Matas, M.; Reynolds, G.K.; Ingram, A. Asymmetric distribution in twin screw granulation. Eur. J. Pharm. Biopharm. 2016, 106, 50–58. [Google Scholar] [CrossRef]
- Silva, A.F.T.; Burggraeve, A.; Denon, Q.; Van Der Meeren, P.; Sandler, N.; Van Den Kerkhof, T.; Hellings, M.; Vervaet, C.; Remon, J.P.; Lopes, J.A.; et al. Particle sizing measurements in pharmaceutical applications: Comparison of in-process methods versus off-line methods. Eur. J. Pharm. Biopharm. 2013, 85, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Portier, C.; Vigh, T.; Di Pretoro, G.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Continuous twin screw granulation: Impact of binder addition method and surfactants on granulation of a high-dosed, poorly soluble API. Int. J. Pharm. 2020, 577, 119068. [Google Scholar] [CrossRef]
- Dhenge, R.M.; Fyles, R.S.; Cartwright, J.J.; Doughty, D.G.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Granule properties. Chem. Eng. J. 2010, 164, 322–329. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Janssens, L.; Vercruysse, J.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous twin screw granulation of controlled release formulations with various HPMC grades. Int. J. Pharm. 2016, 511, 1048–1057. [Google Scholar] [CrossRef] [Green Version]
- Saleh, M.F.; Dhenge, R.M.; Cartwright, J.J.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Effect of process and formulation variables on powder caking during production. Int. J. Pharm. 2015, 496, 571–582. [Google Scholar] [CrossRef]
- Ryckaert, A.; Stauffer, F.; Vanhoorne, V.; Vervaet, C.; Beer, T. De Investigating the use of torque as in-process control for granule size during twin screw wet granulation. unpublished work.
- Verstraeten, M.; Van Hauwermeiren, D.; Lee, K.; Turnbull, N.; Wilsdon, D.; am Ende, M.; Doshi, P.; Vervaet, C.; Brouckaert, D.; Mortier, S.T.F.C.; et al. In-depth experimental analysis of pharmaceutical twin-screw wet granulation in view of detailed process understanding. Int. J. Pharm. 2017, 529, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Vanhoorne, V.; Bekaert, B.; Peeters, E.; De Beer, T.; Remon, J.P.; Vervaet, C. Improved tabletability after a polymorphic transition of delta-mannitol during twin screw granulation. Int. J. Pharm. 2016, 506, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fell, J.T.; Newton, J.M. Tablet Strength by the Diametral-Compression Test. J. Pharm. Sci. 1970, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Flory, J.H.; Panmai, S.; Batra, U.; Kaufman, M.J. Wettability of pharmaceutical solids: Its measurement and influence on wet granulation. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 206, 547–554. [Google Scholar] [CrossRef]
- Grymonpré, W.; Verstraete, G.; Van Bockstal, P.J.; Van Renterghem, J.; Rombouts, P.; De Beer, T.; Remon, J.P.; Vervaet, C. In-line monitoring of compaction properties on a rotary tablet press during tablet manufacturing of hot-melt extruded amorphous solid dispersions. Int. J. Pharm. 2017, 517, 348–358. [Google Scholar] [CrossRef] [Green Version]
- De Backere, C.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Evaluation of an external lubrication system implemented in a compaction simulator. Int. J. Pharm. 2020, 587, 119675. [Google Scholar] [CrossRef]
- Keleb, E.I.; Vermeire, A.; Vervaet, C.; Remon, J.P. Continuous twin screw extrusion for the wet granulation of lactose. Int. J. Pharm. 2002, 239, 69–80. [Google Scholar] [CrossRef]
- Van Melkebeke, B.; Vervaet, C.; Remon, J.P. Validation of a continuous granulation process using a twin-screw extruder. Int. J. Pharm. 2008, 356, 224–230. [Google Scholar] [CrossRef]
- Vandevivere, L.; Portier, C.; Vanhoorne, V.; Häusler, O.; Simon, D.; De Beer, T.; Vervaet, C. Native starch as in situ binder for continuous twin screw wet granulation. Int. J. Pharm. 2019, 571, 118760. [Google Scholar] [CrossRef] [PubMed]
- Iveson, S.M.; Litster, J.D.; Hapgood, K.; Ennis, B.J. Nucleation, growth and breakage phenomena in agitated wet granulation processes: A review. Powder Technol. 2001, 117, 3–39. [Google Scholar] [CrossRef]
- Van Snick, B.; Holman, J.; Cunningham, C.; Kumar, A.; Vercruysse, J.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous direct compression as manufacturing platform for sustained release tablets. Int. J. Pharm. 2017, 519, 390–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jachowicz, R. Dissolution rates of partially water-soluble drugs from solid dispersion systems. I. Prednisolone. Int. J. Pharm. 1987, 35, 1–5. [Google Scholar] [CrossRef]
- Rades, T.; Knopp, M.M.; Olesen, N.E.; Holm, P.E.R.; Langguth, P.; Ren, E. Influence of Polymer Molecular Weight on Drug – Polymer Solubility: A Comparison between Experimentally Determined Solubility in PVP and Prediction Derived from Solubility in Monomer. J. Pharm. Sci. 2015, 2905–2912. [Google Scholar] [CrossRef] [Green Version]
- Rowe, R.C.R.; Sheskey, P.J.S.; Cook, W. Handbook Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press and the American Pharmacists Association: Washington, DC, USA, 2009; ISBN 978-0-12-088479-7. [Google Scholar]
- Kumar, A.; Vercruysse, J.; Toiviainen, M.; Panouillot, P.E.; Juuti, M.; Vanhoorne, V.; Vervaet, C.; Remon, J.P.; Gernaey, K.V.; De Beer, T.; et al. Mixing and transport during pharmaceutical twin-screw wet granulation: Experimental analysis via chemical imaging. Eur. J. Pharm. Biopharm. 2014, 87, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Cantor, S.L.; Augsburger, L.L.; Hoag, S.W.; Gerhardt, A. Pharmaceutical granulation processes, mechanism, and the use of binders. In Pharmaceutical Dosage Forms: Tablets, Third Edition: Volume 1: Unit Operations and Mechanical Properties; Informa Healthcare: London, UK, 2008; pp. 261–302. ISBN 9781420020281. [Google Scholar]
- Sabri, A.H.; Hallam, C.N.; Baker, N.A.; Murphy, D.S.; Gabbott, I.P. Understanding tablet defects in commercial manufacture and transfer. J. Drug Deliv. Sci. Technol. 2018, 46, 1–6. [Google Scholar] [CrossRef]
- Pitt, K.G.; Heasley, M.G. Determination of the tensile strength of elongated tablets. Powder Technol. 2013, 238, 169–175. [Google Scholar] [CrossRef]
- Pitt, K.G.; Webber, R.J.; Hill, K.A.; Dey, D.; Gamlen, M.J. Compression prediction accuracy from small scale compaction studies to production presses. Powder Technol. 2015, 270, 490–493. [Google Scholar] [CrossRef]









| Binder | L/S-Ratio Used for Granulation |
|---|---|
| PVP K12 | 0.180, 0.205, 0.230, 0.255 |
| PVP K90 | 0.130, 0.155, 0.180, 0.205 |
| HPMC E15 | 0.130, 0.155, 0.180, 0.205 |
| PVA 4-88 | 0.105, 0.130 |
| Maltodextrin 6 | 0.180, 0.205, 0.230 |
| SOS CO 01 | 0.105, 0.130, 0.155 |
| HP pea starch | 0.130, 0.155, 0.180, 0.205 |
| Binder | Characterization Technique | Abbreviation Characterization Technique Used in PCA_2 |
|---|---|---|
| HP pea starch (1,2) | Particle size distribution (1) | |
| HPMC E15 (1,2) | Dissolution kinetics (1,2) | DissRate_30, DissRate_60, DissRate_90 |
| HPMC E5 (1) | CA binder solution measured on PTFE (1) | |
| Maltodextrin 2 (1) | CA binder solution measured on mannitol tablet (2) | CAMannitol_t0, CAMannitol_t30 |
| Maltodextrin 6 (1,2) | CA measured on tablet binder (2) | CAbinder_t0, CAbinder_t30 |
| Maltodextrin DSH (1) | Dynamic viscosity (1,2) | Dynamic Viscosity |
| PVA 4-88 (1,2) | Viscosity slope (1,2) | ViscositySlope |
| PVP K12 (1,2) | Surface Tension (1,2) | ST |
| PVP K30 (1) | ||
| PVP K90 (1,2) | ||
| SOS CO 01 (1,2) |
| Binder | L/S-Ratio | Granule Friability (%) | Torque Values (Nm) | PCA Label Color (Symbol) |
|---|---|---|---|---|
| PVP K12 | 0.0675 | 21.7 (±1.0) | 6.1 (±0.6) | Green |
| Maltodextrin 6 | 0.0675 | 21.4 (±1.8) | 7.2 (±0.4) | Green |
| HP pea starch | 0.0675 | 23.5 (±0.8) | 9.5 (±0.8) | Green |
| PVA 4-88 | 0.0800 | 25.9 (±1.1) | 0.7 (±0.2) | Red (circle) |
| SOS CO 01 | 0.0800 | 22.0 (±1.3) | 5.4 (±0.3) | Red (circle) |
| PVP K90 | 0.0800 | 21.7 (±0.1) | 7.4 (±0.5) | Red (triangle) |
| HPMC E15 | 0.1050 | 19.3 (±1.9) | 4.9 (±0.3) | Blue |
| Binder Characteristic (Units) | Dissolution Kinetics (%) | Wettability (°) | Surface Tension (mN/m) | Dynamic Viscosity (mPa·s) | Viscosity Slope | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbreviation | DissRate _30 | DissRate _60 | DissRate _90 | CAbinder _t0 | CAbinder _t30 | CAMannitol _t0 | CAMannitol _t30 | CAPTFE _t0 | CADCP _t0 | ST | Dynamic Viscosity | Viscosity Slope |
| Binder | ||||||||||||
| PVP K12 | 9.6 | 9.9 | 9.9 | NA * | NA * | 39.8 (±2.0) | 26.9 (±1.5) | 96.4 (±1.5) | 109.6 (±3.1) | 50.7 (±0.5) | 1.58 (±0.01) | 0.040 |
| PVP K90 | 3.0 | 4.0 | 6.6 | 76.7 (±2.9) | 56.0 (±1.0) | 55.4 (±1.9) | 38.0 (±0.9) | 98.2 (±1.5) | 84.7 (±1.3) | 44.7 (±0.4) | 99.48 (±1.55) | 0.175 |
| HPMC E15 | 0.5 | 2.5 | 2.5 | 79.1 (±1.7) | 70.2 (±1.8) | 69.4 (±2.6) | 51.3 (±0.9) | 82.1 (±1.1) | 69.6 (±4.4) | 49.0 (±0.2) | 817.10 (±2.75) | 0.300 |
| PVA 4-88 | 5.0 | 6.3 | 7.0 | 67.5 (±1.3) | 48.1 (±0.8) | 54.4 (±1.2) | 34.6 (±1.7) | 82.9 (±0.4) | 36.8 (±2.5) | 43.2 (±0.2) | 13.08 (±0.16) | 0.119 |
| Maltodextrin 6 | 9.7 | 10.0 | 10.0 | 60.0 (±3.9) | 47.8 (±2.1) | 40.9 (±2.5) | 35.4 (±7.8) | 95.4 (±1.0) | 103.0 (±5.3) | 66.8 (±0.2) | 2.07 (±0.07) | 0.053 |
| SOS CO 01 | 3.8 | 6.1 | 6.1 | 68.7 (±2.0) | 47.2 (±0.5) | 47.3 (±2.5) | 33.9 (±2.1) | 78.0 (±0.6) | 32.8 (±3.9) | 30.2 (±0.2) | 4.05 (±0.03) | 0.085 |
| HP pea starch | 8.7 | 10.0 | 10.0 | 67.0 (±3.8) | 57.5 (±4.6) | 43.7 (±0.5) | 33.0 (±0.5) | 94.9 (±0.8) | 95.3 (±0.5) | 57.7 (±0.5) | 15.67 (±0.45) | 0.110 |
| Binder | Plasticity Factor (%) |
|---|---|
| PVP K12 | 94.72 (±0.26) |
| PVP K90 | 94.75 (±0.09) |
| HPMC E15 | 88.01 (±0.08) |
| PVA 4-88 | 94.68 (±0.04) |
| Maltodextrin 6 | 95.69 (±0.01) |
| SOS CO 01 | 96.15 (±0.04) |
| HP pea starch | 96.38 (±0.05) |
| Binder | L/S Mannitol | L/S DCP | Friability Mannitol (%) | Friability DCP (%) |
|---|---|---|---|---|
| PVP K12 | 0.0675 | 0.2050 | 11.7 | 19.0 |
| PVP K90 | 0.0800 | 0.1550 | 9.9 | 2.8 |
| HPMC E15 | 0.1050 | 0.1800 | 19.3 | 2.2 |
| PVA 4-88 | 0.0800 | 0.1300 | 3.0 | 5.9 |
| Maltodextrin 6 | 0.0675 | 0.2300 | 11.1 | 28.9 |
| SOS CO 01 | 0.0800 | 0.1300 | 2.2 | 14.0 |
| HP pea starch | 0.0675 | 0.1800 | 9.7 | 7.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandevivere, L.; Vangampelaere, M.; Portier, C.; de Backere, C.; Häusler, O.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Identifying Critical Binder Attributes to Facilitate Binder Selection for Efficient Formulation Development in a Continuous Twin Screw Wet Granulation Process. Pharmaceutics 2021, 13, 210. https://doi.org/10.3390/pharmaceutics13020210
Vandevivere L, Vangampelaere M, Portier C, de Backere C, Häusler O, De Beer T, Vervaet C, Vanhoorne V. Identifying Critical Binder Attributes to Facilitate Binder Selection for Efficient Formulation Development in a Continuous Twin Screw Wet Granulation Process. Pharmaceutics. 2021; 13(2):210. https://doi.org/10.3390/pharmaceutics13020210
Chicago/Turabian StyleVandevivere, Lise, Maxine Vangampelaere, Christoph Portier, Cedrine de Backere, Olaf Häusler, Thomas De Beer, Chris Vervaet, and Valérie Vanhoorne. 2021. "Identifying Critical Binder Attributes to Facilitate Binder Selection for Efficient Formulation Development in a Continuous Twin Screw Wet Granulation Process" Pharmaceutics 13, no. 2: 210. https://doi.org/10.3390/pharmaceutics13020210
APA StyleVandevivere, L., Vangampelaere, M., Portier, C., de Backere, C., Häusler, O., De Beer, T., Vervaet, C., & Vanhoorne, V. (2021). Identifying Critical Binder Attributes to Facilitate Binder Selection for Efficient Formulation Development in a Continuous Twin Screw Wet Granulation Process. Pharmaceutics, 13(2), 210. https://doi.org/10.3390/pharmaceutics13020210