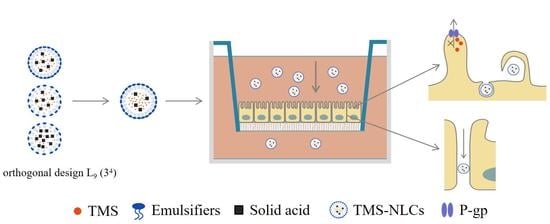
Optimization of Tilmicosin-Loaded Nanostructured Lipid Carriers Using Orthogonal Design for Overcoming Oral Administration Obstacle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation Optimization of TMS-NLCs
2.2.1. Preparation of TMS-NLCs
2.2.2. Orthogonal Experimental Design
2.3. Characterization of TMS-NLCs
2.3.1. Hydrodynamic Diameters, Polydispersity Index, and Zeta Potentials of TMS-NLCs
2.3.2. The Morphology of TMS-NLCs
2.3.3. Entrapment Efficiency and Drug Loading of TMS-NLCs
2.3.4. In Vitro Drug Release of TMS-NLCs
2.3.5. High-Speed Centrifugal Stability
2.3.6. HPLC Analysis of TMS
2.4. Validation of Cell Monolayer Models
2.4.1. The Culture of Cell Monolayers
2.4.2. Integrity of Cell Monolayers
2.4.3. Transmission Electron Microscopy of Monolayers
2.4.4. Permeability of Cell Monolayers
2.5. Effect of NLCs and Endocytosis Inhibitors on Cell Viability
2.6. The Permeability of TMS-NLCs across MDCK-chAbcg2/Abcb1 Cell Monolayers
2.7. Transport Mechanism of TMS-NLCs across MDCK-chAbcg2/abcb1 Monolayers
2.8. Antibacterial Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. The Optimal Preparation Conditions
3.2. Optimization and Characterization of TMS-NLCs
3.3. High-Speed Centrifugal Stability
3.4. Validation of Cell Monolayer Models
3.4.1. Integrity of Cell Monolayers
3.4.2. Transmission Electron Microscopy of Monolayers
3.4.3. Permeability of Cell Monolayers
3.5. Effect of NLCs and Endocytosis Inhibitors on Cell Viability
3.6. The Permeability of TMS-NLCs across MDCK-chAbcg2/Abcb1 Cell Monolayers
3.7. Transport Mechanism of TMS-NLCs across MDCK-chAbcg2/Abcb1 Cell Monolayers
3.8. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef] [Green Version]
- Pucek, A.; Tokarek, B.; Waglewska, E.; Bazylińska, U. Recent advances in the structural design of photosensitive agent formulations using “soft” colloidal nanocarriers. Pharmaceutics 2020, 12, 587. [Google Scholar] [CrossRef]
- Chen, C.; Lee, Y.; Chang, S.; Tsai, Y.; Fang, J.; Hwang, T. Oleic acid-loaded nanostructured lipid carrier inhibit neutrophil activities in the presence of albumin and alleviates skin inflammation. Int. J. Nanomed. 2019, 14, 6539–6553. [Google Scholar] [CrossRef] [Green Version]
- Jnaidi, R.; Almeida, A.J.; Gonçalves, L.M. Solid lipid nanoparticles and nanostructured lipid carriers as smart drug delivery systems in the treatment of Glioblastoma Multiforme. Pharmaceutics 2020, 12, 860. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnol. Adv. 2020, 38, 107287. [Google Scholar] [CrossRef] [PubMed]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed Nanotechnol. 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Gaba, B.; Fazil, M.; Ali, A.; Baboota, S.; Sahni, J.K.; Ali, J. Nanostructured lipid (NLCs) carriers as a bioavailability enhancement tool for oral administration. Drug Deliv. 2015, 22, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Ziv, G.; Shem-Tov, M.; Glickman, A.; Winkler, M.; Saran, A. Tilmicosin antibacterial activity and pharmacokinetics in cows. J. Vet. Pharmacol. Ther. 1995, 18, 340–345. [Google Scholar] [CrossRef] [PubMed]
- IbrahimA, E.; Abdel-Daim, M.M. Modulating effects of Spirulina platensis against tilmicosin-induced cardiotoxicity in mice. Cell J. 2015, 17, 137–144. [Google Scholar]
- Al-Qushawi, A.; Rassouli, A.; Atyabi, F.; Peighambari, S.M.; Esfandyari-Manesh, M.; Shams, G.R.; Yazdani, A. Preparation and characterization of three tilmicosin-loaded lipid nanoparticles: Physicochemical properties and in-vitro antibacterial activities. Iran. J. Pharm. Res. 2016, 15, 663–676. [Google Scholar] [PubMed]
- Vogel, G.J.; Laudert, S.B.; Zimmermann, A.; Guthrie, C.A.; Mechor, G.D.; Moore, G.M. Effects of tilmicosin on acute undifferentiated respiratory tract disease in newly arrived feedlot cattle. JAVMA J. Am. Vet. Med. A 1998, 212, 1919–1924. [Google Scholar]
- Xie, S.; Wang, F.; Wang, Y.; Zhu, L.; Dong, Z.; Wang, X.; Li, X.; Zhou, W. Acute toxicity study of tilmicosin-loaded hydrogenated castor oil-solid lipid nanoparticles. Part. Fibre. Toxicol. 2011, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Zhu, Q.; Zhao, Y.; Yang, S.; Cao, J.; Qiu, Y. Tilmicosin enteric granules and premix to pigs: Antimicrobial susceptibility testing and comparative pharmacokinetics. J. Vet. Pharmacol. Ther. 2019, 42, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Li, Y.; Fai, C.; Cui, S.; Cui, B. The controlled release of tilmicosin from silica nanoparticles. Drug Dev. Ind. Pharm. 2011, 37, 714. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, X.; Chen, D.; Yuan, Y.; Wang, S.; Li, C.; Yan, Y.; Liu, Q.; Shao, L.; Huang, L.; et al. Enhanced treatment effects of tilmicosin against Staphylococcus aureus cow mastitis by self-assembly sodium alginate-chitosan nanogel. Pharmaceutics 2019, 11, 524. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Wang, M.; Bhutto, R.A.; Zhao, H.; Cohen Stuart, M.A.; Wang, J. Facile preparation of tilmicosin-loaded polymeric nanoparticle with controlled properties and functions. ACS Omega 2020, 5, 32366–32372. [Google Scholar] [CrossRef]
- Sahito, B.; Zhang, Q.; Yang, H.; Peng, L.; Gao, X.; Kashif, J.; Aabdin, Z.U.; Jiang, S.; Wang, L.; Guo, D. Synthesis of tilmicosin nanostructured lipid carriers for improved oral delivery in broilers: Physiochemical characterization and cellular permeation. Molecules 2020, 25, 315. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yang, H.; Sahito, B.; Li, X.; Peng, L.; Gao, X.; Ji, H.; Wang, L.; Jiang, S.; Guo, D. Nanostructured lipid carriers with exceptional gastrointestinal stability and inhibition of P-gp efflux for improved oral delivery of tilmicosin. Colloid Surf. B 2020, 187, 110649. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zhang, Z.; Wang, T. Preparation and heat-insulating properties of Al2O3–ZrO2 (Y2O3) hollow fibers derived from cogon using an orthogonal experimental design. RSC Adv. 2019, 9, 11305. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huang, J.; Liu, Y.; Guo, T.; Wang, L. Using the lentiviral vector system to stably express chicken P-gp and BCRP in MDCK cells for screening the substrates and studying the interplay of both transporters. Arch. Toxicol. 2018, 92, 2027–2042. [Google Scholar] [CrossRef]
- Zhang, Q.; Sahito, B.; Li, L.; Peng, L.; Jiang, S.; Guo, D. Determination of encapsulation efficacy and loading capacity of tilmicosin loaded nanostructured lipid carriers by ultrafiltration centrifugation combined with high performance liquid chromatography. Anim. Hus. Vet. Med. 2019, 51, 56–61. [Google Scholar]
- Neves, A.R.; Queiroz, J.F.; Lima, S.A.C.; Figueiredo, F.; Fernandes, R.; Reis, S. Cellular uptake and transcytosis of lipid-based nanoparticles across the intestinal barrier: Relevance for oral drug delivery. J. Colloid Interf. Sci. 2016, 463, 258–265. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Gascón, A.R.; Pozo-Rodríguez, A.D.; Rieux, A.D.; Préat, V. Mechanism of transport of saquinavir-loaded nanostructured lipid carriers across the intestinal barrier. J. Control Release 2013, 166, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.; Jia, Z.; Du, W.; Yu, C.; Fan, Y.; Dai, W.; Yuan, L.; Zhang, H.; Wang, X.; Wang, J.; et al. The transport pathways of polymer nanoparticles in MDCK epithelial cells. Biomaterials 2013, 34, 4309–4326. [Google Scholar] [CrossRef]
- Wassermann, L.; Halwachs, S.; Lindner, S.; Honscha, K.U.; Honscha, W. Determination of functional ABCG2 activity and assessment of drug-ABCG2 interactions in dairy animals using a novel MDCKII in vitro model. J. Pharm. Sci. 2013, 102, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Roger, E.; Lagarce, F.; Garcion, E.; Benoit, J.P. Lipid nanocarriers improve paclitaxel transport throughout human intestinal epithelial cells by using vesicle-mediated transcytosis. J. Control Release 2009, 140, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.; Xu, Y.; Chen, S.; Cheng, B.; Hu, F.; You, J.; Du, Y.; Yuan, H. Transport mechanisms of solid lipid nanoparticles across Caco-2 cell monolayers and their related cytotoxicology. ACS Appl. Mater. Inter. 2016, 8, 5929–5940. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Swami, R.; Khan, W.; Sistla, R. Lymphatic system: A prospective area for advanced targeting of particulate drug carriers. Expert Opin. Drug Del. 2014, 11, 211–229. [Google Scholar] [CrossRef]
- He, Z.; Hu, Y.; Nie, T.; Tang, H.; Zhu, J.; Chen, K.; Liu, L.; Leong, K.W.; Chen, Y.; Mao, H. Size-controlled lipid nanoparticle production using turbulent mixing to enhance oral DNA delivery. Acta Biomater. 2018, 81, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tsai, T.; Huang, Z.; Fang, J. Effects of lipophilic emulsifiers on the oral administration of lovastatin from nanostructured lipid carriers: Physicochemical characterization and pharmacokinetics. Eur. J. Pharm. Biopharm. 2010, 74, 474–482. [Google Scholar] [CrossRef]
- Tiwari, R.; Pathak, K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: Comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int. J. Pharmaceut. 2011, 415, 232–243. [Google Scholar] [CrossRef] [PubMed]
- ElShaer, A.; Mustafa, S.; Kasar, M.; Thapa, S.; Ghatora, B.; Alany, R.G. Nanoparticle-laden contact lens for controlled ocular delivery of prednisolone: Formulation optimization using statistical experimental design. Pharmaceutics 2016, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shen, L.; Wang, T.; Li, H.; Huang, R.; Zhang, Z.; Wang, Y.; Quan, D. Taste masking of water-soluble drug by solid lipid microspheres: A child-friendly system established by reversed lipid-based nanoparticle technique. J. Pharm. Pharmacol. 2020, 72, 776–786. [Google Scholar] [CrossRef]
- Wang, T.; Xue, J.; Hu, Q.; Zhou, M.; Luo, Y. Preparation of lipid nanoparticles with high loading capacity and exceptional gastrointestinal stability for potential oral delivery applications. J. Colloid Interf. Sci. 2017, 507, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Karsten, M. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliver. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Bazylińska, U.; Kulbacka, J.; Chodaczek, G. Nanoemulsion Structural Design in Co-Encapsulation of Hybrid Multifunctional Agents: Influence of the Smart PLGA Polymers on the Nanosystem-Enhanced Delivery and Electro-Photodynamic Treatment. Pharmaceutics 2019, 11, 405. [Google Scholar] [CrossRef] [Green Version]
- Shakeel, F.; Ramadan, W. Transdermal delivery of anticancer drug caffeine from water-in-oil nanoemulsions. Colloid Surface B 2009, 75, 356–362. [Google Scholar] [CrossRef]
- Yan, G.; Liang, Q.; Wen, X.; Peng, J.; Deng, R.; Lv, L.; Ji, M.; Deng, X.; Wu, L.; Feng, X.; et al. Preparation, characterization, and pharmacokinetics of tilmicosin taste-masked formulation via hot-melt extrusion technology. Colloid Surf. B 2020, 196, 111293. [Google Scholar] [CrossRef] [PubMed]
- Ayehunie, S.; Islam, A.; Cannon, C.; Landry, T.; Pudney, J.; Klausner, M.; Anderson, D.J. Characterization of a hormone-responsive organotypic human vaginal tissue model: Morphologic and immunologic effects. Reprod. Sci. 2015, 22, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, J.M.; Leong, K.W. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv. Drug Deliver. Rev. 2013, 65, 800–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, M.J.; Thompson, D.P.; Cramer, C.T.; Vidmar, T.J.; Scieszka, J.F. The Madin Darby canine kidney (MDCK) epithelial cell monolayer as a model cellular transport barrier. Pharm. Res. 1989, 6, 71–77. [Google Scholar] [CrossRef]
- Yang, X.; Yang, X.; Wang, Y.; Ma, L.; Zhang, Y.; Yang, X.; Wang, K. Establishment of Caco-2 cell monolayer model and standard operation procedure for assessing intestinal absorption of chemical components of traditional Chinese medicine. J. Chin. Integr. Med. 2007, 5, 634–641. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.H.; Yu, A. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef]
- National Archives & Records Service of Office. FDA draft guidance for industry on drug interaction studies-study design, data analysis, implications for dosing, and labeling recommendations. Availab. Fed. Regist. 2012, 77, 9946. [Google Scholar]
- Ma, B.; Wang, J.; Sun, J.; Li, M.; Xu, H.; Sun, G.; Sun, X. Permeability of rhynchophylline across human intestinal cell in vitro. Int. J. Clin. Exp. Patho. 2014, 7, 1957–1966. [Google Scholar]
- Chen, X.; Wang, T.; Lu, M.; Zhu, L.; Wang, Y.; Zhou, W. Preparation and evaluation of tilmicosin-loaded hydrogenated castor oil nanoparticle suspensions of different particle sizes. Int. J. Nanomed. 2014, 9, 2655–2664. [Google Scholar]





| Levels | Factors | |||
|---|---|---|---|---|
| A (g/g) | B (wt %) | C (wt %) | D (mL/mL) | |
| 1 | 1:9 | 20 | 10 | 2:1 |
| 2 | 1:6 | 25 | 20 | 1:1 |
| 3 | 1:3 | 30 | 30 | 1:2 |
| No. | A (g/g) | B (wt %) | C (wt %) | D (mL/mL) | HD (nm) | EE (%) | DL (%) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 344.67 ± 18.50 | 90.39 ± 0.092 | 2.68 ± 0.03 |
| 2 | 1 | 2 | 2 | 2 | 342.60 ± 10.94 | 90.02 ± 0.64 | 6.08 ± 0.04 |
| 3 | 1 | 3 | 3 | 3 | 553.73 ± 25.37 | 93.60 ± 0.3 | 9.49 ± 0.03 |
| 4 | 2 | 1 | 2 | 3 | 405.67 ± 13.65 | 90.69 ± 0.67 | 5.24 ± 0.04 |
| 5 | 2 | 2 | 3 | 1 | 534.47 ± 29.11 | 95.00 ± 0.13 | 8.56 ± 0.01 |
| 6 | 2 | 3 | 1 | 2 | 292.50 ± 1.65 | 91.59 ± 0.39 | 3.39 ± 0.01 |
| 7 | 3 | 1 | 3 | 2 | 595.30 ± 33.99 | 93.74 ± 0.52 | 8.54 ± 0.05 |
| 8 | 3 | 2 | 1 | 3 | 346.60 ± 5.90 | 91.72 ± 0.30 | 3.60 ± 0.01 |
| 9 | 3 | 3 | 2 | 1 | 407.57 ± 32.38 | 94.26 ± 0.24 | 6.38 ± 0.02 |
| HD | |||||||
| K1 | 413.67 | 448.55 | 327.92 | 428.90 | |||
| K2 | 410.88 | 407.89 | 385.28 | 410.13 | |||
| K3 | 449.82 | 417.93 | 561.17 | 435.33 | |||
| R | 38.94 | 40.66 | 233.24 | 25.20 | |||
| EE | |||||||
| K1 | 91.34 | 91.61 | 91.23 | 93.22 | |||
| K2 | 92.43 | 92.25 | 91.66 | 91.78 | |||
| K3 | 93.24 | 93.15 | 94.11 | 92.00 | |||
| R | 1.90 | 1.54 | 2.88 | 1.43 | |||
| DL | |||||||
| K1 | 6.08 | 5.49 | 3.22 | 5.87 | |||
| K2 | 5.73 | 6.08 | 5.90 | 6.00 | |||
| K3 | 6.17 | 6.42 | 8.86 | 6.11 | |||
| R | 0.44 | 0.93 | 5.64 | 0.24 | |||
| Evaluating Indicator | Primary and Secondary Order | Optimal Formulations |
|---|---|---|
| HD | C > B > A > D | A2B2C1D2 |
| EE | C > A > B > D | A3B3C3D1 |
| DL | C > B > A > D | A3B3C3D3 |
| No. | Formulations | HD (nm) | PDI | ZP (mV) | EE (%) | DL (%) |
|---|---|---|---|---|---|---|
| 1 | A1B3C1D1 | 283.70 ± 4.10 | 0.249 ± 0.059 | −32.75 ± 1.63 | 89.21 ± 1.10 | 3.04 ± 0.04 |
| 2 | A1B3C1D2 | 273.30 ± 10.04 | 0.288 ± 0.071 | −32.90 ± 1.84 | 91.50 ± 1.65 | 3.38 ± 0.06 |
| 3 | A1B2C2D1 | 292.15 ± 22.42 | 0.192 ± 0.031 | −33.50 ± 0.57 | 88.50 ± 0.96 | 4.69 ± 0.05 |
| 4 | A1B2C2D3 | 281.60 ± 6.22 | 0.337 ± 0.033 | −33.70 ± 0.71 | 87.56 ± 0.33 | 5.60 ± 0.02 |
| 5 | A1B3C2D1 | 277.10 ± 11.31 | 0.264 ± 0.067 | −31.10 ± 0.99 | 91.26 ± 0.21 | 6.71 ± 0.02 |
| 6 | A1B3C2D2 | 264.60 ± 10.32 | 0.291 ± 0.011 | −33.70 ± 0.42 | 89.54 ± 1.09 | 5.57 ± 0.07 |
| 7 | A1B3C2D3 | 272.40 ± 2.55 | 0.339 ± 0.023 | −33.65 ± 0.21 | 88.05 ± 0.94 | 6.75 ± 0.07 |
| 8 | A1B3C3D1 | 268.20 ± 9.19 | 0.239 ± 0.059 | −29.60 ± 0.99 | 92.51 ± 0.08 | 8.66 ± 0.01 |
| 9 | A1B3C3D2 | 276.85 ± 2.62 | 0.231 ± 0.001 | −31.10 ± 0.00 | 92.92 ± 0.42 | 9.14 ± 0.04 |
| 10 | A1B3C3D3 | 279.10 ± 2.40 | 0.252 ± 0.042 | −30.65 ± 0.35 | 87.01 ± 1.02 | 8.20 ± 0.10 |
| 11 | A3B3C2D1 | 291.05 ± 0.07 | 0.232 ± 0.001 | −29.25 ± 1.06 | 92.94 ± 0.62 | 7.25 ± 0.05 |
| 12 | A3B3C2D2 | 306.30 ± 4.81 | 0.268 ± 0.000 | −29.60 ± 0.14 | 90.11 ± 1.05 | 6.54 ± 0.08 |
| 13 | A3B3C2D3 | 309.65 ± 3.18 | 0.260 ± 0.007 | −30.20 ± 0.14 | 87.42 ± 0.23 | 6.40 ± 0.02 |
| 14 | A3B3C3D1 | 336.75 ± 13.51 | 0.238 ± 0.081 | −29.20 ± 2.26 | 93.93 ± 0.62 | 9.51 ± 0.06 |
| 15 | A3B3C3D2 | 316.30 ± 2.69 | 0.310 ± 0.042 | −29.80 ± 1.41 | 90.13 ± 1.03 | 8.15 ± 0.09 |
| 16 | A2B2C1D2 | 305.79 ± 16.18 | 0.290 ± 0.019 | −31.49 ± 0.86 | 96.78 ± 0.63 | 2.77 ± 0.19 |
| 17 | A1B3C1D3 | 294.02 ± 11.29 | 0.372 ± 0.023 | −31.27 ± 0.57 | 94.59 ± 0.57 | 2.49 ± 0.06 |
| 18 | A1B2C2D2 | 362.20 ± 8.64 | 0.267 ± 0.008 | −32.51 ± 0.37 | 96.41 ± 0.19 | 5.45 ± 0.15 |
| 19 | A3B3C3D3 | 449.61 ± 5.78 | 0.391 ± 0.055 | −27.77 ± 0.72 | 91.68 ± 0.55 | 8.56 ± 0.17 |
| Conditions | HD (nm) | PDI | ZP (mV) | KE | Precipitate | |
|---|---|---|---|---|---|---|
| Untreatment | 278.93 ± 5.59 | 0.215 ± 0.035 | −30.20 ± 0.89 | - | - | |
| 4 °C | 5 min | 219.30 ± 4.24 | 0.251 ± 0.008 | −30.10 ± 1.27 | 0.212 ± 0.014 | - |
| 10 min | 219.05 ± 2.19 | 0.270 ± 0.042 | −29.70 ± 1.13 | 0.213 ± 0.007 | - | |
| 20 min | 198.50 ± 2.12 | 0.384 ± 0.096 | −31.05 ± 0.35 | 0.286 ± 0.007 | + | |
| 30 min | 194.70 ± 2.26 | 0.198 ± 0.002 | −29.95 ± 0.21 | 0.299 ± 0.007 | + | |
| 25 °C | 5 min | 196.60 ± 4.81 | 0.365 ± 0.033 | −29.05 ± 0.35 | 0.292 ± 0.016 | - |
| 10 min | 193.95 ± 15.20 | 0.399 ± 0.062 | −30.50 ± 0.57 | 0.304 ± 0.049 | + | |
| 20 min | 193.70 ± 11.46 | 0.254 ± 0.037 | −28.90 ± 0.57 | 0.302 ± 0.037 | + | |
| 30 min | 200.90 ± 7.07 | 0.277 ± 0.049 | −29.20 ± 0.99 | 0.278 ± 0.023 | + | |
| Tracers | Concentration (µM) | Papp (×10−6 cm/s) | |
|---|---|---|---|
| MDCK | MDCK-chAbcg2/Abcb1 | ||
| Lucifer yellow | 225 | 0.22 ± 0.06 | 0.13 ± 0.01 |
| Propranolol | 100 | 16.17 ± 0.65 | 17.61 ± 0.64 |
| Formulations | Verapamil | MDCK | MDCK-chAbcg2/Abcb1 | NER | ||||
|---|---|---|---|---|---|---|---|---|
| Papp (×10−6 cm/s) | ER | Papp (×10−6 cm/s) | ER | |||||
| AP→BL | BL→AP | AP→BL | BL→AP | |||||
| API | − | 0.42 ± 0.04 | 0.53 ± 0.02 | 1.27 | 0.40 ± 0.02 | 1.06 ± 0.03 | 2.63 | 2.07 |
| + | 0.38 ± 0.02 | 0.41 ± 0.04 | 1.06 | 0.39 ± 0.04 | 0.39 ± 0.07 ** | 1.00 | 0.94 | |
| TMS-NLCs | − | 0.48 ± 0.01 | 0.51 ± 0.02 | 1.07 | 0.59 ± 0.05 ** | 0.96 ± 0.11 | 1.62 | 1.51 |
| + | 0.49 ± 0.06 | 0.47 ± 0.05 | 0.96 | 0.36 ± 0.04 | 0.36 ± 0.08 ** | 1.00 | 1.04 | |
| Formulations | Strains | |||
|---|---|---|---|---|
| S. aureus | S. agalactiae | E. coli | S. Typhimurium | |
| API | <0.5 | 16 | 16 | 16 |
| TMS-NLCs | <0.5 | 16 | 16 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Gao, X.; Zhang, Q.; Sahito, B.; Si, H.; Li, G.; Ding, Q.; Wu, W.; Nepovimova, E.; Jiang, S.; et al. Optimization of Tilmicosin-Loaded Nanostructured Lipid Carriers Using Orthogonal Design for Overcoming Oral Administration Obstacle. Pharmaceutics 2021, 13, 303. https://doi.org/10.3390/pharmaceutics13030303
Wen J, Gao X, Zhang Q, Sahito B, Si H, Li G, Ding Q, Wu W, Nepovimova E, Jiang S, et al. Optimization of Tilmicosin-Loaded Nanostructured Lipid Carriers Using Orthogonal Design for Overcoming Oral Administration Obstacle. Pharmaceutics. 2021; 13(3):303. https://doi.org/10.3390/pharmaceutics13030303
Chicago/Turabian StyleWen, Jia, Xiuge Gao, Qian Zhang, Benazir Sahito, Hongbin Si, Gonghe Li, Qi Ding, Wenda Wu, Eugenie Nepovimova, Shanxiang Jiang, and et al. 2021. "Optimization of Tilmicosin-Loaded Nanostructured Lipid Carriers Using Orthogonal Design for Overcoming Oral Administration Obstacle" Pharmaceutics 13, no. 3: 303. https://doi.org/10.3390/pharmaceutics13030303
APA StyleWen, J., Gao, X., Zhang, Q., Sahito, B., Si, H., Li, G., Ding, Q., Wu, W., Nepovimova, E., Jiang, S., Wang, L., Kuca, K., & Guo, D. (2021). Optimization of Tilmicosin-Loaded Nanostructured Lipid Carriers Using Orthogonal Design for Overcoming Oral Administration Obstacle. Pharmaceutics, 13(3), 303. https://doi.org/10.3390/pharmaceutics13030303