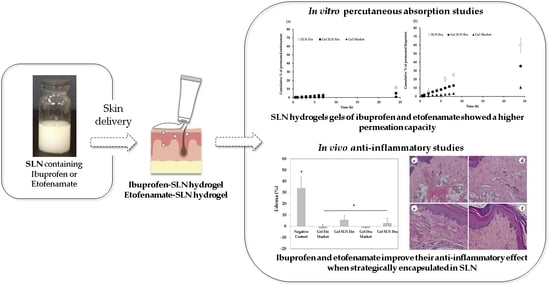
Increased Therapeutic Efficacy of SLN Containing Etofenamate and Ibuprofen in Topical Treatment of Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. SLN Preparation
2.2. Physical Characterization of SLNs
2.2.1. Particle Size and Distribution
2.2.2. Zeta Potential
2.3. Thermal Analysis
2.3.1. Differential Scanning Calorimetry (DSC)
2.3.2. Dynamic Light Scattering (DLS)
2.4. Transmission Electron Microscopy (TEM)
2.5. Atomic Force Microscopy (AFM)
2.6. Encapsulation Efficiency
2.7. Etofenamate and Ibuprofen Quantification
2.8. In Vitro Cytotoxicity and Internalization Studies
2.9. Preparation of Hydrogels
2.10. Characterization of the Hydrogels
2.11. Stability Studies
2.12. In Vitro Studies of Percutaneous Absorption
2.12.1. Release Studies
- (1)
- Zero-order model: Q1= Q0 + K0t, where Q1 is the amount of drug dissolved in time t, Q0 is the initial amount of drug in solution, and K0 é is the zero-order release constant;
- (2)
- Higuchi model: Qt= K √t, where Qt is the amount of drug released in time t, and K is the Higuchi release constant.
2.12.2. Permeation Studies
2.13. Anti-Inflammatory Effect of Ibuprofen-SLN and Etofenamate-SLN Gels
2.14. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Etofenamate and Ibuprofen-Loaded SLN
3.2. DSC Thermal Analysis
3.3. DLS Thermal Analysis
3.4. Morphological Characterization
3.5. In Vitro Cytotoxicity and Internalization Studies
3.6. Drug-Loaded Hydrogel Physicochemical Characterization, Microbiological Efficacy, and Stability
3.7. In Vitro Studies of Percutaneous Absorption
3.8. In Vivo Evaluation of the Anti-Inflammatory Effect of Etofenamate-SLN Sel and Ibuprofen-SLN Gel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watanabe, T.; Fujiwara, Y.; Chan, F.K.L. Current knowledge on non-steroidal anti-inflammatory drug-induced small-bowel damage: A comprehensive review. J. Gastroenterol. 2020, 55, 481–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villar, M.A.; Pagan, J.A.; Palacios, L.; Quiralte, J.; Ramirez, M. Allergic contact dermatitis to etofenamate. Cross-reaction to other non-steroid anti-inflammatory drugs. Contact Dermat. 2008, 58, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Raposo, S.; Simões, S.; Almeida, A.J.; Ribeiro, H. Advanced vehicles for topical delivery of glucocorticoids. Exp. Opin. Drug Deliv. 2013, 10, 857–877. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Pasula, N.; Kumar, R.; Chowdary, P. Piroxicam proliposomal gel—A novel approach for topical delivery. J. Pharm. Res. 2012, 5, 1755–1763. [Google Scholar]
- Beloqui, A.; Solinís, M.A.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Chenreddy, S.; Wang, J.; Prabhu, S. Evaluation of ibuprofen loaded solid lipid nanoparticles and its combination regimens for pancreatic cancer chemoprevention. Int. J. Oncol. 2015, 46, 1827–1834. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A.; Neumann, D.; Lamprecht, A. Lipid nanocapsules for dermal application: A comparative study of lipid-based versus polymer-based nanocarriers. Eur. J. Pharm. Biopharm. 2011, 79, 36–42. [Google Scholar] [CrossRef]
- Gumustas, M.; Sengel-Turk, C.T.; Badilli, U.; Amasya, G.; Ozkan, S.A.; Tarimci, N. Optimization of stability indicating LC method for the sensitive in vitro determination from Solid Lipid Nanoparticles and ex vivo analysis from rat skin of etofenamate. Curr. Pharm. Anal. 2017, 13, 63. [Google Scholar] [CrossRef]
- Uner, M.; Damgalı, S.; Ozdemir, S.; Celik, B. Therapeutic potential of drug delivery by means of lipid nanoparticles: Reality or illusion? Curr. Pharm. Des. 2017. [Google Scholar] [CrossRef]
- Liu, M.; Wen, J.; Sharma, M. Solid lipid nanoparticles for topical drug delivery: Mechanisms, dosage form perspectives, and translational status. Curr. Pharm. Des. 2020, 26, 3203–3217. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, K.; Anbu, J.; Ravichandiran, V.; Venkateswarlu, V.; Rao, Y.M. Lipid nanoparticles for transdermal delivery of flurbiprofen: Formulation, in vitro, ex vivo and in vivo studies. Lipids Health Dis. 2009, 8, 6–21. [Google Scholar] [CrossRef] [Green Version]
- Khurana, S.; Bedi, P.M.S.; Jain, N.K. Preparation and evaluation of solid lipid nanoparticles based nanogel for dermal delivery of meloxicam. Chem. Phys. Lipids 2013, 175–176, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Colaço, R.; Gonçalves, M.C.; Fortes, L.M.; Gonçalves, L.M.D.; Almeida, A.J.; Martins, B.F. Preparation and chemical characterization of eco-friendly ORMOSIL nanoparticles of potential application in DNA gene therapy. Curr. Nanosci. 2013, 9, 168–172. [Google Scholar]
- Cadete, A.; Figueiredo, L.; Lopes, R.; Calado, C.C.R.; Almeida, A.J.; Gonçalves, L.M.D. Development and characterization of a new plasmid delivery system based on chitosansodium deoxycholate nanoparticles. Eur. J. Pharm. Sci. 2012, 45, 451–458. [Google Scholar] [CrossRef]
- Lopes, R.; Eleutério, C.V.; Gonçalves, L.M.D.; Cruz, M.E.M.; Almeida, A.J. Lipid nanoparticles containing oryzalin for the treatment of leishmaniasis. Eur. J. Pharm. Sci. 2012, 45, 442–450. [Google Scholar] [CrossRef]
- Vitorino, C.; Almeida, J.; Gonçalves, L.M.D.; Almeida, A.J.; Sousa, J.J.; Pais, A.A.C.C. Coencapsulating nanostructured lipid carriers for transdermal application: From experimental design to the molecular detail. J. Control. Release 2013, 167, 301–314. [Google Scholar] [CrossRef]
- European Pharmacopoeia (Ph. Eur.), 9th ed.; Council of Europe: Strasbourg, France, 2017.
- Guidance for Industry Q1A(R2) Stability Testing of New Drug Substances and Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-r2-stability-testing-new-drug-substances-products-step-5_en.pdf (accessed on 20 December 2020).
- Costa, P.; Lobo, G.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Naito, Y.; Yasumuro, M.; Kondou, K.; Ohara, N. Anti-inflammatory effect of topically applied propolis extract in carrageenan-induced rat hind paw edema. Phytother. Res. 2007, 21, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Bagde, A.; Patel, K.; Kutlehria, S.; Chowdhury, N.; Singh, M. Formulation of topical ibuprofen solid lipid nanoparticle (SLN) gel using hot melt extrusion technique (HME) and determining its anti-inflammatory strength. Drug Deliv. Transl. Res. 2019, 9, 816–827. [Google Scholar] [CrossRef]
- Pham, C.V.; Van, M.C.; Thic, H.P.; Thanh, C.Đ.; Ngoc, B.T.; Van, B.N.; Thien, G.L.; Van, B.N.; Nguyen, C.N. Development of ibuprofen-loaded solid lipid nanoparticle-based hydrogels for enhanced in vitro dermal permeation and in vivo topical an-ti-inflammatory activity. J. Drug Deliv. Sci. Technol. 2020, 57, 101758. [Google Scholar] [CrossRef]
- Casadei, M.A.; Cerreto, F.; Cesa, S.; Giannuzzo, M.; Feeney, M.; Marianecci, C.; Paolicelli, P. Solid lipid nanoparticles incorporated in dextran hydrogels: A new drug delivery system for oral formulations. Int. J. Pharm. 2006, 325, 140–146. [Google Scholar] [CrossRef]
- Zimmermann, E.; Souto, E.B.; Muller, R.H. Physicochemical investigations on the structure of drug-free and drug-loaded solid lipid nanoparticles (SLNTM) by means of DSC and 1H NMR. Pharmazie 2005, 60, 508–513. [Google Scholar]
- Gaspar, D.P.; Faria, V.; Gonçalves, L.M.; Taboada, P.; Remuñán-López, C.; Almeida, A.J. Rifabutin-loaded solid lipid nanoparticles for inhaled antitubercular therapy: Physicochemical and in vitro studies. Int. J. Pharm. 2016, 497, 199–209. [Google Scholar] [CrossRef]
- Üner, M. Characterization and Imaging of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- OECD. OECD Guideline for the Testing of Chemicals (No. 439.): In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD: Paris, France, 2015. [Google Scholar]
- Doktorovova, S.; Kovacevic, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Küchler, S.; Radowski, M.R.; Blaschke, T.; Dathe, M.; Plendl, J.; Haag, R.; Schäfer-Korting, M.; Kramer, K.D. Nanoparticles for skin penetration enhancement—A comparison of adendritic core-multishell-nanotransporter and solid lipid nanoparticles. Eur. J. Pharm. Biopharm. 2009, 71, 243–250. [Google Scholar] [CrossRef]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Effect of propylene glycol on ibuprofen absorption into human skin in vivo. J. Pharm. Sci. 2008, 97, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Lopes, R.M.; Clemente, P.; Raposo, S.; Gonçalves, L.M.D.; Bica, A.; Ribeiro, H.M.; Almeida, A.J. Lecithin and parabens play a crucial role in tripalmitin-based lipid nanoparticle stabilization throughout moist heat sterilization and freeze-drying. Eur. J. Lipid Sci. Technol. 2015, 117, 1947–1959. [Google Scholar] [CrossRef]
- Marto, J.; Baltazar, D.; Militão, M.; Duarte, A.; Salgado, A.; Gouveia, L.; Simões, S.; Fernandes, A.; Oliveira, E.; Ribeiro, H. Topical gels of etofenamate: In vitro and in vivo evaluation. Pharm. Dev. Technol. 2014, 5, 710–715. [Google Scholar]
- Mateus, D.; Marto, J.; Trindade, P.; Gonçalves, H.; Salgado, A.; Machado, P.; Melo-Gouveia, A.; Ribeiro, H.M.; Almeida, A.J. Improved morphine-loaded hydrogels for wound-related pain relief. Pharmaceutics 2019, 11, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelli, F.; Puglia, C.; Sanpietro, M.G.; Rizza, L.; Bonina, F. Characterization of indomethacin-loaded lipid nanoparticles by differential scanning calorimetry. Int. J. Pharm. 2005, 304, 231–238. [Google Scholar] [CrossRef]
- Available online: www.chemspider.com (accessed on 14 December 2020).
- Hadgraft, J.; Plesis, J.; Goosen, C. The selection of non-steroidal anti-inflammatory agents for dermal delivery. Int. J. Pharm. 2000, 207, 31–37. [Google Scholar] [CrossRef]
- Fang, J.Y.; Fang, C.; Liu, C.; Su, Y. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2008, 70, 633–640. [Google Scholar] [CrossRef]
- Venkateswarlu, V.; Manjunath, K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparti-cles. J. Control. Release 2004, 95, 627–638. [Google Scholar] [CrossRef]
- Vaghasiya, H.; Kumar, A.; Sawant, K. Development of solid lipid nanoparticles based controlled release system for topical delivery of terbinafine hydrochloride. Eur. J. Pharm. Sci. 2013, 49, 311–322. [Google Scholar] [CrossRef]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Enhanced permeation of fentanyl from supersaturated solutions in a model membrane. Int. J. Pharm. 2011, 407, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Influence of penetration enhancer on drug permeation from volatile for-mulations. Int. J. Pharm. 2012, 439, 260–268. [Google Scholar] [CrossRef]
- Kogan, A.; Gart, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef]
- Trotta, M.; Ugazio, E.; Peira, E.; Pulitano, C. Influence of ion pairing on topical delivery of retinoic acid from microemulsions. J. Control. Release 2003, 86, 315–321. [Google Scholar] [CrossRef]
- Dias, M.; Hadgraft, J.; Lane, M.E. Influence of membrane-solvent-solute interactions on solute permeation in model mem-branes. Int. J. Pharm. 2007, 336, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.; Hadgraft., J.; Lane, M.E. Influence of membrane-solvent-solute interactions on solute permeation in skin. Int. J. Pharm. 2007, 340, 65–70. [Google Scholar] [CrossRef]
- Cross, S.E.; Magnusson, B.M.; Winckle, G.; Anissimov, Y.; Roberts, M.S. Determination of the effect of lipophilicity on the in vitro permeability and tissue reservoir characteristics of topically applied solutes in human skin layers. J. Investig. Dermatol. 2003, 120, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.J.; Toscano, C.; Videira, M. Therapeutic applications of lipid nanoparticles administered by alternative routes. In Proceedings of the XII International Workshop on Bioencapsulation, Vitória, Spain, 24–26 September 2004; Pedraz, J.L., Orive, G., Poncelet, D., Eds.; pp. 69–72. [Google Scholar]
- Komatsu, T.; Sakurada, T. Comparison of the efficacy and skin permeability of topical NSAID preparations used in Europe. Eur. J. Pharm. Sci. 2012, 47, 890–895. [Google Scholar] [CrossRef]
- Watkinson, R.M.; Herkenne, C.; Guy, R.H.; Hadgraft, J.; Oliveira, G.; Lane, M.E. Influence of ethanol on the solubility, ioniza-tion and permeation charactheristics of ibuprofen in silicone and human skin. Skin Pharmacol. Physiol. 2009, 22, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Hayashi, T.; Kawabata, S.; Seki, T.; Sugibayashi, K. Effect of l-mentholethanol-water system on the systemic absorption of flurbiprofen after repeated dermal applications in rabbits. Biol. Pharm. Bull. 2000, 23, 1254–1257. [Google Scholar] [CrossRef] [Green Version]
- Vitorino, C.; Alves, L.; Antunes, F.E.; Sousa, J.J.; Pais, A.A.C.C. Design of a dual nanostructured lipid carrier formulation based on physicochemical, rheological, and mechanical properties. J. Nanopart. Res. 2013, 15, 1993. [Google Scholar] [CrossRef]
- Badilli, U.; Sengel-Turk, C.T.; Onay-Besikci, A.; Tarimci, N. Development of etofenamate-loaded semisolid SLN dispersions and evaluation of anti-inflammatory activity for topical application. Curr. Drug Deliv. 2015, 12, 200–209. [Google Scholar] [CrossRef]
- Badilli, U.; Sengel-Turk, C.T.; Amasya, G.; Tarimci, N. Novel drug delivery system for dermal uptake of etofenamate: Semisolid SLN dispersion. Curr. Drug Deliv. 2017, 14, 386–393. [Google Scholar] [CrossRef]
- Lucetti, D.L.; Lucetti, E.C.; Bandeira, M.; Veras, H.N.; Silva, A.H.; Leal, L.K.; Lopes, A.A.; Alves, V.C.; Silva, G.S.; Brito, G.A.; et al. Anti-inflammatory effects and possible mechanism of action of lupeol acetate isolated from Himatanthus drasticus (Mart). Plumel. J. Inflamm. 2010, 7, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, A.H.; Duarte, N.; Serra, A.T.; Ferreira, A.; Bronze, M.R.; Custódio, L.; Gaspar, M.M.; Simões, S.; Rijo, P.; Ascensão, L.; et al. Further evidence of possible therapeutic uses of Sambucus nigra L. extracts by the assessment of the in vitro and in vivo anti-inflammatory properties of its PLGA and PCL-based nanoformulations. Pharmaceutics 2020, 12, 1181. [Google Scholar] [CrossRef] [PubMed]












| Formulation | Compritol® 888 ATO (%) | Tween® 80 (%) | HPMC (%) | Propylene glycol (%) | Ethyl alcohol (%) | Menthol (%) | Eto (%) | Ibu (%) | Water (%) |
|---|---|---|---|---|---|---|---|---|---|
| Gel SLN | 2.0 | 0.6 | 2.0 | 10.0 | 10.0 | 0.1 | qs 100 | ||
| Gel SLN Eto | 2.0 | 0.6 | 2.0 | 10.0 | 10.0 | 0.1 | 0.6 | qs 100 | |
| Gel SLN Ibu | 2.0 | 0.6 | 2.0 | 10.0 | 10.0 | 0.1 | 0.3 | qs 100 |
| Time (months) | Gel SLN Eto | Gel SLN Ibu | ||||
|---|---|---|---|---|---|---|
| Recovery of Etofenamate (%) | pH | Viscosity (Pa.s) | Recovery of Ibuprofen (%) | pH | Viscosity (Pa.s) | |
| Batches stored at room temperature (25 ± 2 °C/60 ± 5% RH) | ||||||
| 0 | 98.97 ± 0.65 | 5.60 ± 0.11 | 41,133 ± 1.80 | 100.27 ± 0.85 | 5.64 ± 0.05 | 46,133 ± 2.30 |
| 1 | 100.89 ± 2.06 | 5.59 ± 0.12 | 46,866 ± 3.20 | 98.31 ± 0.52 | 5.59 ± 0.03 | 46,000 ± 7.20 |
| 3 | 99.70 ± 1.63 | 5.59 ± 0.15 | 43,133 ± 5.70 | 102.39 ± 0.37 | 5.64 ± 0.03 | 45,933 ± 12.5 |
| 6 | 103.73 ± 1.00 | 5.42 ± 0.11 | 42,400 ± 6.50 | 98.00 ± 4.12 | 5.67 ± 0.06 | 38,600 ± 3.70 |
| 12 | 100.50 ± 2.72 | 5.12 ± 0.16 | 26,966 ± 2.60 | 95.09 ± 1.37 | 5.61 ± 0.04 | 30,600 ± 24.2 |
| Batches stored at intermediate conditions (30 ± 2 °C/60 ± 5% RH) | ||||||
| 0 | 98.97 ± 0.65 | 5.60 ± 0.11 | 41,133 ± 1.80 | 100.27 ± 0.85 | 5.64 ± 0.05 | 46,133 ± 2.30 |
| 1 | 101.38 ± 0.84 | 5.41 ± 0.07 | 46,133 ± 4.30 | 97.36 ± 0.81 | 4.41 ± 0.07 | 45,133 ± 3.90 |
| 3 | 100.22 ± 2.80 | 5.46 ± 0.17 | 40,466 ± 8.30 | 102.11 ± 2.39 | 4.34 ± 0.04 | 41,233 ± 4.06 |
| 6 | 104.17 ± 0.57 | 5.50 ± 0.02 | 43,466 ± 7.70 | 89.11 ± 4.48 | 4.40 ± 0.02 | 34,466 ± 2.89 |
| 12 | -- | -- | -- | -- | -- | -- |
| Batches stored in at accelerated conditions (40 ± 2 °C/75 ± 5% RH) | ||||||
| 0 | 98.97 ± 0.65 | 5.60 ± 0.11 | 41,133 ± 1.80 | 100.27 ± 0.85 | 5.64 ± 0.05 | 46,133 ± 2.30 |
| 1 | 98.99 ± 0.02 | 5.43 ± 0.07 | 38,600 ± 4.40 | 96.07 ± 2.66 | 4.43 ± 0.07 | 47,200 ± 4.20 |
| 3 | 100.50 ± 2.95 | 5.46 ± 0.14 | 38,033 ± 2.40 | 102.07 ± 3.74 | 4.04 ± 0.57 | 37,866 ± 8.20 |
| 6 | 99.38 ± 3.83 | 5.42 ± 0.01 | 38,733 ± 1.10 | 91.81 ± 5.56 | 4.42 ± 0.01 | 34,200 ± 2.00 |
| 12 | -- | -- | -- | -- | -- | -- |
| Formulation | Kp (cm h−1) | J (µg cm−2h−1) | TL (h) |
|---|---|---|---|
| Etofenamate-SLNs | 3.12 × 10−3 | 3.74 | 3.05 |
| Etofenamate-SLN hydrogel | 5.12 × 10−3 | 6.15 | 1.23 |
| Etofenamate reference gel | 2.35 × 10−3 | 11.97 | 0.65 |
| Ibuprofen-SLNs | 5.05 × 10−3 | 4.83 | 4.04 |
| Ibuprofen-SLN hydrogel | 1.16 × 10−2 | 17.55 | 0.20 |
| Ibuprofen reference gel | 2.98 × 10−2 | 22.85 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, G.; Gonçalves, L.M.D.; Marto, J.; Carvalho, F.A.; Simões, S.; Ribeiro, H.M.; Almeida, A.J. Increased Therapeutic Efficacy of SLN Containing Etofenamate and Ibuprofen in Topical Treatment of Inflammation. Pharmaceutics 2021, 13, 328. https://doi.org/10.3390/pharmaceutics13030328
Mancini G, Gonçalves LMD, Marto J, Carvalho FA, Simões S, Ribeiro HM, Almeida AJ. Increased Therapeutic Efficacy of SLN Containing Etofenamate and Ibuprofen in Topical Treatment of Inflammation. Pharmaceutics. 2021; 13(3):328. https://doi.org/10.3390/pharmaceutics13030328
Chicago/Turabian StyleMancini, Giuliana, Lídia M. D. Gonçalves, Joana Marto, Filomena A. Carvalho, Sandra Simões, Helena Margarida Ribeiro, and António J. Almeida. 2021. "Increased Therapeutic Efficacy of SLN Containing Etofenamate and Ibuprofen in Topical Treatment of Inflammation" Pharmaceutics 13, no. 3: 328. https://doi.org/10.3390/pharmaceutics13030328
APA StyleMancini, G., Gonçalves, L. M. D., Marto, J., Carvalho, F. A., Simões, S., Ribeiro, H. M., & Almeida, A. J. (2021). Increased Therapeutic Efficacy of SLN Containing Etofenamate and Ibuprofen in Topical Treatment of Inflammation. Pharmaceutics, 13(3), 328. https://doi.org/10.3390/pharmaceutics13030328