Optimising an Infusion Protocol Containing Cefepime to Limit Particulate Load to Newborns in a Neonatal Intensive Care Unit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiments, Devices and Drugs
2.2. pH Measurement
2.3. Osmolality Measurement
2.4. Static Particle Load Analysis
2.5. Dynamic Particle Load Analysis
2.6. Evaluation of the Origin of Particles and Proposed Strategy to Reduce Particulate Load
2.6.1. Modalities for Reconstituting/Diluting Cefepime
2.6.2. Study of Cefepime/Vancomycin Interaction
2.6.3. Adding an Additional Filter
2.7. Determination of Cefepime Dihydrochloride Monohydrate Concentration
2.8. Statistics
3. Results
3.1. pH and Osmolality Measurements
3.2. Static Particle Counting
3.3. Dynamic Measurement of Particulate Contamination during Infusion
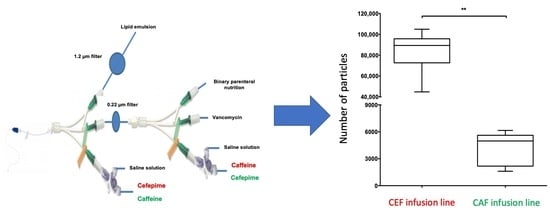
3.3.1. Comparison of the Particulate Load of CEF and CAF Infusion Lines
3.3.2. Comparison of Particulate Load between the CEF Infusion Line with Vancomycin and without
3.3.3. Comparison of Particulate Load between the CEF, CEF with Filter and CAF Infusion Lines
3.4. Impact of the Filter on Cefepime Dihydrochloride Monohydrate Concentration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taxis, K.; Barber, N. Incidence and Severity of Intravenous Drug Errors in a German Hospital. Eur. J. Clin. Pharmacol. 2004, 59, 815–817. [Google Scholar] [CrossRef]
- Tissot, E.; Cornette, C.; Demoly, P.; Jacquet, M.; Barale, F.; Capellier, G. Medication Errors at the Administration Stage in an Intensive Care Unit. Intensive Care Med. 1999, 25, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.S.; Kuhlman, J.E.; Voytovich, M. Microvascular Pulmonary Emboli Secondary to Precipitated Crystals in a Patient Receiving Total Parenteral Nutrition: A Case Report and Description of the High-Resolution CT Findings. Chest 1999, 115, 892–895. [Google Scholar] [CrossRef]
- Monte, S.V.; Prescott, W.A.; Johnson, K.K.; Kuhman, L.; Paladino, J.A. Safety of Ceftriaxone Sodium at Extremes of Age. Expert Opin. Drug Saf. 2008, 7, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S.; Wassel, R.T.; Lee, L.; Nambiar, S. Intravenous Ceftriaxone and Calcium in the Neonate: Assessing the Risk for Cardiopulmonary Adverse Events. Pediatrics 2009, 123, e609–e613. [Google Scholar] [CrossRef] [PubMed]
- Jack, T.; Boehne, M.; Brent, B.E.; Hoy, L.; Köditz, H.; Wessel, A.; Sasse, M. In-Line Filtration Reduces Severe Complications and Length of Stay on Pediatric Intensive Care Unit: A Prospective, Randomized, Controlled Trial. Intensive Care Med. 2012, 38, 1008–1016. [Google Scholar] [CrossRef] [Green Version]
- Boehne, M.; Jack, T.; Köditz, H.; Seidemann, K.; Schmidt, F.; Abura, M.; Bertram, H.; Sasse, M. In-Line Filtration Minimizes Organ Dysfunction: New Aspects from a Prospective, Randomized, Controlled Trial. BMC Pediatr. 2013, 13, 21. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.E.; Heldman, L.S.; Goo, E.D.; Whippo, P.E.; Perkinson, J.C. Fatal Microvascular Pulmonary Emboli from Precipitation of a Total Nutrient Admixture Solution. JPEN J. Parenter. Enteral Nutr. 1996, 20, 81–87. [Google Scholar] [CrossRef]
- McNearney, T.; Bajaj, C.; Boyars, M.; Cottingham, J.; Haque, A. Total Parenteral Nutrition Associated Crystalline Precipitates Resulting in Pulmonary Artery Occlusions and Alveolar Granulomas. Dig. Dis. Sci. 2003, 48, 1352–1354. [Google Scholar] [CrossRef]
- Leff, R.D.; Roberts, R.J. Practical Aspects of Intravenous Drug Administration: Principles and Techniques for Nurses, Pharmacists and Physicians, 2nd ed.; American Society of Hospital Pharmacists’ Special Projects Division: Bethesda, MD, USA, 1992. [Google Scholar]
- Gikic, M.; Di Paolo, E.R.; Pannatier, A.; Cotting, J. Evaluation of Physicochemical Incompatibilities during Parenteral Drug Administration in a Paediatric Intensive Care Unit. Pharm. World Sci. 2000, 22, 88–91. [Google Scholar] [CrossRef]
- Kalikstad, B.; Skjerdal, A.; Hansen, T.W.R. Compatibility of Drug Infusions in the NICU. Arch. Dis. Child. 2010, 95, 745–748. [Google Scholar] [CrossRef]
- Rounds, I.V. What You Should Know about Drug Compatibility. Nursing 2008, 38, 15. [Google Scholar] [CrossRef]
- Council of Europe. Particulate Contamination: Sub-Visible Particles. In The European Pharmacopoeia, 9th ed.; EDQM Council of Europe: Strasbourg, France, 2019; Chapter 2.9.19. [Google Scholar]
- Foinard, A.; Décaudin, B.; Barthélémy, C.; Debaene, B.; Odou, P. The Impact of Multilumen Infusion Devices on the Occurrence of Known Physical Drug Incompatibility: A Controlled in Vitro Study. Anesth. Analg. 2013, 116, 101–106. [Google Scholar] [CrossRef]
- Perez, M.; Décaudin, B.; Abou Chahla, W.; Nelken, B.; Barthélémy, C.; Lebuffe, G.; Odou, P. In Vitro Analysis of Overall Particulate Contamination Exposure during Multidrug IV Therapy: Impact of Infusion Sets. Pediatr. Blood Cancer 2015, 62, 1042–1047. [Google Scholar] [CrossRef]
- Perez, M.; Décaudin, B.; Foinard, A.; Barthélémy, C.; Debaene, B.; Lebuffe, G.; Odou, P. Compatibility of Medications during Multi-Infusion Therapy: A Controlled in Vitro Study on a Multilumen Infusion Device. Anaesth. Crit. Care Pain Med. 2015, 34, 83–88. [Google Scholar] [CrossRef]
- Jaimovich, D.G.; Rose, W.W. In Vivo Evaluation of Simultaneous Administration of Incompatible Drugs via a Double-Lumen Peripheral Catheter. Crit. Care Med. 1990, 18, 1164–1166. [Google Scholar] [CrossRef]
- Collins, J.L.; Lutz, R.J. In Vitro Study of Simultaneous Infusion of Incompatible Drugs in Multilumen Catheters. Heart Lung 1991, 20, 271–277. [Google Scholar]
- Ball, P.A. Intravenous In-Line Filters: Filtering the Evidence. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 319–325. [Google Scholar] [CrossRef]
- Van Lingen, R.A.; Baerts, W.; Marquering, A.C.M.; Ruijs, G.J.H.M. The Use of In-Line Intravenous Filters in Sick Newborn Infants. Acta Paediatr. 2004, 93, 658–662. [Google Scholar] [CrossRef]
- Perez, M.; Décaudin, B.; Abou Chahla, W.; Nelken, B.; Storme, L.; Masse, M.; Barthélémy, C.; Lebuffe, G.; Odou, P. Effectiveness of In-Line Filters to Completely Remove Particulate Contamination during a Pediatric Multidrug Infusion Protocol. Sci. Rep. 2018, 8, 7714. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogen, A.; Krediet, T.G.; Uiterwaal, C.S.P.M.; Bolenius, J.F.G.A.; Gerards, L.J.; Fleer, A. In-Line Filters in Central Venous Catheters in a Neonatal Intensive Care Unit. J. Perinat. Med. 2006, 34, 71–74. [Google Scholar] [CrossRef]
- Niël-Weise, B.S.; Stijnen, T.; van den Broek, P.J. Should In-Line Filters Be Used in Peripheral Intravenous Catheters to Prevent Infusion-Related Phlebitis? A Systematic Review of Randomized Controlled Trials. Anesth. Analg. 2010, 110, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.; Richards, R.; Showell, M. Intravenous In-Line Filters for Preventing Morbidity and Mortality in Neonates. Cochrane Database Syst. Rev. 2006, CD005248. [Google Scholar] [CrossRef] [Green Version]
- Virlouvet, A.-L.; Pansiot, J.; Toumazi, A.; Colella, M.; Capewell, A.; Guerriero, E.; Storme, T.; Rioualen, S.; Bourmaud, A.; Biran, V.; et al. In-Line Filtration in Very Preterm Neonates: A Randomized Controlled Trial. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [Green Version]
- Trissel, L.A. Handbook on Injectable Drugs; American Society of Health-System Pharmacists: Bethesda, MD, USA, 2011. [Google Scholar]
- Stabilis 4.0. Available online: https://www.stabilis.org/# (accessed on 17 May 2019).
- Raverdy, V.; Ampe, E.; Hecq, J.-D.; Tulkens, P.M. Stability and Compatibility of Vancomycin for Administration by Continuous Infusion. J. Antimicrob. Chemother. 2013, 68, 1179–1182. [Google Scholar] [CrossRef]
- Berti, A.D.; Hutson, P.R.; Schulz, L.T.; Webb, A.P.; Rose, W.E. Compatibility of Cefepime and Vancomycin during Simulated Y-Site Administration of Prolonged Infusion. Am. J. Health Syst. Pharm. 2015, 72, 390–395. [Google Scholar] [CrossRef]
- Baririan, N.; Chanteux, H.; Viaene, E.; Servais, H.; Tulkens, P.M. Stability and Compatibility Study of Cefepime in Comparison with Ceftazidime for Potential Administration by Continuous Infusion under Conditions Pertinent to Ambulatory Treatment of Cystic Fibrosis Patients and to Administration in Intensive Care Units. J. Antimicrob. Chemother. 2003, 51, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Benlabed, M.; Martin Mena, A.; Gaudy, R.; Perez, M.; Genay, S.; Hecq, J.-D.; Odou, P.; Lebuffe, G.; Décaudin, B. Analysis of Particulate Exposure during Continuous Drug Infusion in Critically Ill Adult Patients: A Preliminary Proof-of-Concept in Vitro Study. Intensive Care Med. Exp. 2018, 6, 38. [Google Scholar] [CrossRef] [Green Version]
- Perez, M.; Décaudin, B.; Maiguy-Foinard, A.; Barthélémy, C.; Lebuffe, G.; Storme, L.; Odou, P. Dynamic Image Analysis to Evaluate Subvisible Particles During Continuous Drug Infusion in a Neonatal Intensive Care Unit. Sci. Rep. 2017, 7, 9404. [Google Scholar] [CrossRef]
- Martin Mena, A.; Masse, M.; Négrier, L.; Carta, N.; Pettinari, A.; Barthélémy, C.; Odou, P.; Genay, S.; Décaudin, B. Dynamic Particle Count during Drug Infusion: Method Characterization and Analysis of Factors Influencing Results. J. Drug Deliv. Sci. Technol. 2020, 55, 101473. [Google Scholar] [CrossRef]
- Lehr, H.-A.; Brunner, J.; Rangoonwala, R.; Kirkpatrick, C.J. Particulate Matter Contamination of Intravenous Antibiotics Aggravates Loss of Functional Capillary Density in Postischemic Striated Muscle. Am. J. Respir. Crit. Care Med. 2002, 165, 514–520. [Google Scholar] [CrossRef]
- Masse, M.; Genay, S.; Mena, A.M.; Carta, N.; Lannoy, D.; Barthélémy, C.; Décaudin, B.; Odou, P. Evaluation of the Stability of Vancomycin Solutions at Concentrations Used in Clinical Services. Eur. J. Hosp. Pharm. 2020. [Google Scholar] [CrossRef]
- Bouchoud, L.; Fonzo-Christe, C.; Klingmüller, M.; Bonnabry, P. Compatibility of Intravenous Medications with Parenteral Nutrition: In Vitro Evaluation. JPEN J. Parenter. Enteral Nutr. 2013, 37, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Stawny, M.; Nadolna, M.; Jelińska, A. In Vitro Compatibility Studies of Vancomycin with Ready-to-Use Parenteral Nutrition Admixtures for Safer Clinical Practice. Clin. Nutr. 2020, 39, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Benlabed, M.; Perez, M.; Gaudy, R.; Genay, S.; Lannoy, D.; Barthélémy, C.; Odou, P.; Lebuffe, G.; Décaudin, B. Clinical Implications of Intravenous Drug Incompatibilities in Critically Ill Patients. Anaesth. Crit. Care Pain Med. 2018. [Google Scholar] [CrossRef]
- Butler, D.L.; Munson, J.M.; DeLuca, P.P. Effect of Inline Filtration on the Potency of Low-Dose Drugs. Am. J. Hosp. Pharm. 1980, 37, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, M.; Rodriguez, C.A.; Zuluaga, A.F.; Vesga, O. Nontherapeutic Equivalence of a Generic Product of Imipenem-Cilastatin Is Caused More by Chemical Instability of the Active Pharmaceutical Ingredient (Imipenem) than by Its Substandard Amount of Cilastatin. PLoS ONE 2019, 14, e0211096. [Google Scholar] [CrossRef] [Green Version]
- Agudelo, M.; Vesga, O. Therapeutic Equivalence Requires Pharmaceutical, Pharmacokinetic, and Pharmacodynamic Identities: True Bioequivalence of a Generic Product of Intravenous Metronidazole. Antimicrob. Agents Chemother. 2012, 56, 2659–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agudelo, M.; Rodriguez, C.A.; Pelaez, C.A.; Vesga, O. Even Apparently Insignificant Chemical Deviations among Bioequivalent Generic Antibiotics Can Lead to Therapeutic Nonequivalence: The Case of Meropenem. Antimicrob. Agents Chemother. 2014, 58, 1005–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Medicines | Protocol Used in NICU | In Vitro Protocol | |||
|---|---|---|---|---|---|
| Reconstitution/Dilution Solutions | Final Concentration (mg/mL) | Infusion Time in the Unit | Protocol Used In Vitro Compared to Clinical Use | Infusion Time In Vitro | |
| Vancomycin | WFI/G5% | 4.1 | 24 h | No change | 8 h |
| Caffeine | G5% | 3.4 | 30 min every 8 or 12 h | No change | 30 min |
| Cefepime | WFI/SS 0.9% | 17.5 | 30 min every 8 or 12 h | No change | 30 min |
| Binary parenteral nutrition | - | - | 24 h | Replaced by G5% | 8 h |
| Lipid emulsion | - | - | 24 h | Replaced by G5% | 8 h |
| SS 0.9% 1 (Rinsing of caffeine) | - | - | 11 min every 8 or 12 h | No change | 11 min |
| SS 0.9% 2 (Rinsing of cefepime) | - | - | 8 min every 8 or 12 h | No change | 8 min |
| Table A | Cefepime (WFI/SS) | Vancomycin (WFI/G5%) | Caffeine (G5%) | G5% | SS | |
|---|---|---|---|---|---|---|
| pH | 4.43 [4.36–4.48] | 3.31 [3.25–3.32] | 2.42 [2.36–2.47] | 3.10 [3.07–3.27] | 5.81 [5.79–5.82] | |
| Osmolality (mOsm/kg) | 380 [380–383] | 252 [249–255] | 276 [271–298] | 293 [292–294] | 285 [284–285] | |
| Table B | Periods T0 to T4H and T4H41 to T8H | Period T4H to T4H30 | Period T4H30 to T4H41 | |||
| pH | 3.41 [3.39–3.46] | 3.52 [3.51–3.52] | 4.20 [4.18–4.21] | |||
| Osmolality (mOsm/kg) | 284 [284–291] | 318 [317–320] | 288 [287–290] | |||
| Medecines (Reconstitution/Dilution Solutions) | ≥10 µm | ≥25 µm |
|---|---|---|
| Vancomycin (WFI/G5%) | 4250 [3300–5920] | 90 [20–230] |
| Caffeine (G5%) | 1746 [556–2188] | 103 [30–182] |
| Cefepime (WFI/SS) | 13,236 [9155–24,239] | 595 [156–1326] |
| Cefepime (WFI/G5%) | 12,534 [9809–25,620] | 1414 [507–2652] |
| Cefepime (SS/SS) | 21,782 [5928–34,613] | 858 [117–5041] |
| Cefepime (G5%/G5%) | 13,638 [6659–23,322] | 410 [67–2057] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin Mena, A.; Masse, M.; Négrier, L.; Nguyen, T.H.; Ladam, B.; Storme, L.; Barthélémy, C.; Odou, P.; Genay, S.; Décaudin, B. Optimising an Infusion Protocol Containing Cefepime to Limit Particulate Load to Newborns in a Neonatal Intensive Care Unit. Pharmaceutics 2021, 13, 351. https://doi.org/10.3390/pharmaceutics13030351
Martin Mena A, Masse M, Négrier L, Nguyen TH, Ladam B, Storme L, Barthélémy C, Odou P, Genay S, Décaudin B. Optimising an Infusion Protocol Containing Cefepime to Limit Particulate Load to Newborns in a Neonatal Intensive Care Unit. Pharmaceutics. 2021; 13(3):351. https://doi.org/10.3390/pharmaceutics13030351
Chicago/Turabian StyleMartin Mena, Anthony, Morgane Masse, Laura Négrier, Thu Huong Nguyen, Bruno Ladam, Laurent Storme, Christine Barthélémy, Pascal Odou, Stéphanie Genay, and Bertrand Décaudin. 2021. "Optimising an Infusion Protocol Containing Cefepime to Limit Particulate Load to Newborns in a Neonatal Intensive Care Unit" Pharmaceutics 13, no. 3: 351. https://doi.org/10.3390/pharmaceutics13030351
APA StyleMartin Mena, A., Masse, M., Négrier, L., Nguyen, T. H., Ladam, B., Storme, L., Barthélémy, C., Odou, P., Genay, S., & Décaudin, B. (2021). Optimising an Infusion Protocol Containing Cefepime to Limit Particulate Load to Newborns in a Neonatal Intensive Care Unit. Pharmaceutics, 13(3), 351. https://doi.org/10.3390/pharmaceutics13030351