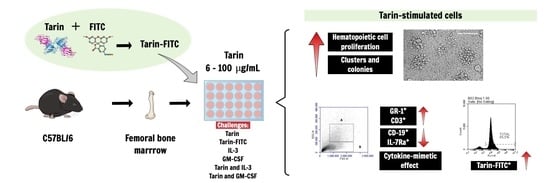
Taro Lectin Can Act as a Cytokine-Mimetic Compound, Stimulating Myeloid and T Lymphocyte Lineages and Protecting Progenitors in Murine Bone Marrow
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Growth Factors
2.2. Tarin Purification
2.3. Mice Bone Marrow Cell Tarin-Binding Evaluations
2.4. Bone Marrow Cell Suspensions and Culture Conditions
2.5. Tarin Effects on Murine Bone Marrow Cell Cultures
2.6. Flow Cytometry Analysis of BM Stimulated with Tarin and/or Growth Factors: Cell Distribution and Phenotype Profile
2.7. Statistical Analyses
3. Results
3.1. Selective Tarin Binding to a Bone Marrow Cell Population
3.2. Tarin Stimulates Bone Marrow Cell Proliferation and Morphological Changes
3.3. Synergic Tarin and Growth Factor Effects on Bone Marrow Cell Cultures
3.4. Tarin and/or Growth Factors Promote Phenotype Changes in BM Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, P.R.; Corrêa, A.C.; Vericimo, M.A.; Paschoalin, V.M. Tarin, a Potential Immunomodulator and COX-Inhibitor Lectin Found in Taro (Colocasia esculenta). Compr. Rev. Food Sci. Food Saf. 2018, 17, 878–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temesgen, M.; Retta, N. Nutritional potential, health and food security benefits of taro Colocasia esculenta (L.): A Review. Food Sci. Qual. Manag. 2015, 36, 23–30. [Google Scholar]
- Siqueira, M.V. Yam: A neglected and underutilized crop in Brazil. Hortic. Bras. 2011, 29, 16–20. [Google Scholar] [CrossRef] [Green Version]
- USDA. Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 17 September 2020).
- Pereira, P.R.; Mattos, É.B.; Corrêa, A.C.; Vericimo, M.A.; Paschoalin, V.M. Anticancer and Immunomodulatory Benefits of Taro (Colocasia esculenta) Corms, an Underexploited Tuber Crop. Int. J. Mol. Sci. 2021, 22, 265. [Google Scholar] [CrossRef]
- Kundu, N.; Campbell, P.; Hampton, B.; Lin, C.-Y.; Ma, X.; Ambulos, N.; Zhao, X.F.; Goloubeva, O.; Holt, D.; Fulton, A.M. Antimetastatic activity isolated from Colocasia esculenta (taro). Anti-Cancer Drugs 2012, 23, 200–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau Sang, C.; Jack Ho, W.; Tzi Bun, N. A Cytokine-Inducing Hemagglutinin from Small Taros. Protein Pept. Lett. 2010, 17, 823–830. [Google Scholar] [CrossRef]
- Correa, A.C.; Vericimo, M.A.; Dashevskiy, A.; Pereira, P.R.; Paschoalin, V.M. Liposomal Taro Lectin Nanocapsules Control Human Glioblastoma and Mammary Adenocarcinoma Cell Proliferation. Molecules 2019, 24, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Ghosh, P.; Das, S. Expression of Colocasia esculenta tuber agglutinin in Indian mustard provides resistance against Lipaphis erysimi and the expressed protein is non-allergenic. Plant Cell Rep. 2018, 37, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Kaur, M.; Kaur, S.; Kaur, A.; Kamboj, S.S.; Singh, J. Purification of Colocasia esculenta lectin and determination of its anti-insect potential towards Bactrocera cucurbitae. J. Environ. Biol. 2013, 34, 31. [Google Scholar] [PubMed]
- Roy, A.; Das, S. Molecular mechanism underlying the entomotoxic effect of Colocasia esculenta tuber agglutinin against Dysdercus cingulatus. Insects 2015, 6, 827–846. [Google Scholar]
- Roy, A.; Banerjee, S.; Majumder, P.; Das, S. Efficiency of mannose-binding plant lectins in controlling a homopteran insect, the red cotton bug. J. Agric. Food Chem. 2002, 50, 6775–6779. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Gupta, S.; Hess, D.; Das, K.P.; Das, S. Binding of insecticidal lectin Colocasia esculenta tuber agglutinin (CEA) to midgut receptors of Bemisia tabaci and Lipaphis erysimi provides clues to its insecticidal potential. Proteomics 2014, 14, 1646–1659. [Google Scholar] [CrossRef]
- Pereira, P.R.; Del Aguila, E.M.; Verícimo, M.A.; Zingali, R.B.; Paschoalin, V.M.; Silva, J.T. Purification and characterization of the lectin from taro (Colocasia esculenta) and its effect on mouse splenocyte proliferation in vitro and in vivo. Protein J. 2014, 33, 92–99. [Google Scholar] [CrossRef]
- Mérida, L.A.; Mattos, É.B.; Corrêa, A.C.; Pereira, P.R.; Paschoalin, V.M.; Pinho, M.F.; Vericimo, M.A. Tarin stimulates granulocyte growth in bone marrow cell cultures and minimizes immunosuppression by cyclo-phosphamide in mice. PLoS ONE 2018, 13, e0206240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keyaerts, E.; Vijgen, L.; Pannecouque, C.; Van Damme, E.; Peumans, W.; Egberink, H.; Balzarini, J.; Van Ranst, M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antivir. Res. 2007, 75, 179–187. [Google Scholar] [CrossRef]
- Pereira, P.R.; Silva, J.T.; Verícimo, M.A.; Paschoalin, V.M.; Teixeira, G.A. Crude extract from taro (Colocasia esculenta) as a natural source of bioactive proteins able to stimulate haematopoietic cells in two murine models. J. Funct. Foods 2015, 18, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, Y.; Wang, K. Comparison of CA125, HE4, and ROMA index for ovarian cancer diagnosis. Curr. Probl. Cancer 2019, 43, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, Z.; Gao, X.; Wu, Q. Advances in the discovery of novel biomarkers for cancer: Spotlight on protein N-glycosylation. Biomark. Med. 2020, 14, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Lee, L.Y.; Kawahara, R.; Abrahams, J.L.; Adamczyk, B.; Anugraham, M.; Ashwood, C.; Sumer-Bayraktar, Z.; Briggs, M.T.; Chik, J.H. Protein Paucimannosylation Is an Enriched N-Glycosylation Signature of Human Cancers. Proteomics 2019, 19, 1900010. [Google Scholar] [CrossRef]
- Tvaroška, I.; Selvaraj, C.; Koča, J. Selectins—The Two Dr. Jekyll and Mr. Hyde Faces of Adhesion Molecules—A Review. Molecules 2020, 25, 2835. [Google Scholar] [CrossRef] [PubMed]
- Soliman, C.; Guy, A.J.; Chua, J.X.; Vankemmelbeke, M.; McIntosh, R.S.; Eastwood, S.; Truong, V.K.; Elbourne, A.; Spendlove, I.; Durrant, L.G. Molecular and structural basis for Lewis glycan recognition by a cancer-targeting antibody. Biochem. J. 2020, 477, 3219–3235. [Google Scholar] [CrossRef]
- Pereira, P.R.; Winter, H.C.; Verícimo, M.A.; Meagher, J.L.; Stuckey, J.A.; Goldstein, I.J.; Paschoalin, V.M.; Silva, J.T. Structural analysis and binding properties of isoforms of tarin, the GNA-related lectin from Colocasia esculenta. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2015, 1854, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Pereira, P.R.; Meagher, J.L.; Winter, H.C.; Goldstein, I.J.; Paschoalin, V.M.; Silva, J.T.; Stuckey, J.A. High-resolution crystal structures of Colocasia esculenta tarin lectin. Glycobiology 2017, 27, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Tanner, R.S.; James, S.A. Rapid bactericidal effect of low pH against Pseudomonas aeruginosa. J. Ind. Microbiol. 1992, 10, 229–232. [Google Scholar] [CrossRef]
- Morton, H.E. Pseudomonas. In Disinfection, Sterilization and Preservation, 3rd ed.; Block, S.S., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1983; pp. 401–413. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- The, T.H.; Feltkamp, T.E. Conjugation of fluorescein isothiocyanate to antibodies. I. Experiments on the conditions of conjugation. Immunology 1970, 18, 865–873. [Google Scholar]
- Goding, J.W. Conjugation of antibodies with fluorochromes: Modifications to the standard methods. J. Immunol. Methods 1976, 13, 215–226. [Google Scholar] [CrossRef]
- Harlow, E.; Lane, D. Labeling antibodies. In Antibodies—A Laboratory Manual, 1st ed.; Harlow, E., Lane, D., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1988; Volume 1, p. 726. [Google Scholar]
- Liu, X.; Quan, N. Immune Cell Isolation from Mouse Femur Bone Marrow. Bio Protoc. 2015, 5, e1631. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Giemsa, G. Färbemethoden für malariaparasiten. Zent. Bakteriol. 1902, 31, 429–430. [Google Scholar]
- May, R.; Grunwald, L. Ueber Blutfarbungen. Zent. Inn. Med. 1902, 23, 265–270. [Google Scholar]
- Nijnik, A. Immunomodulatory approaches for prevention and treatment of infectious diseases. Curr. Opin. Microbiol. 2013, 16, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Vinh, D.C. Cytokine immunomodulation for the treatment of infectious diseases: Lessons from primary immunodeficiencies. Expert Rev. Clin. Immunol. 2014, 10, 1069–1100. [Google Scholar] [CrossRef]
- Locy, H.; de Mey, S.; de Mey, W.; De Ridder, M.; Thielemans, K.; Maenhout, S.K. Immunomodulation of the Tumor Microenvironment: Turn Foe Into Friend. Front. Immunol. 2018, 9, 2909. [Google Scholar] [CrossRef]
- Serrano-del Valle, A.; Naval, J.; Anel, A.; Marzo, I. Novel Forms of Immunomodulation for Cancer Therapy. Trends Cancer 2020, 6, 518–532. [Google Scholar] [CrossRef]
- Borriello, F.; Galdiero, M.R.; Varricchi, G.; Loffredo, S.; Spadaro, G.; Marone, G. Innate Immune Modulation by GM-CSF and IL-3 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 834. [Google Scholar] [CrossRef] [Green Version]
- Pawar, H.; Choudhary, P.D.; Kamat, S. An Overview of Traditionally Used Herb, Colocasia esculenta, as a Phytomedicine. Med. Aromat. Plants 2018, 7, 1–5. [Google Scholar] [CrossRef]
- Sudhakar, P.; Thenmozhi, V.; Srivignesh, S.; Dhanalakshmi, M. Colocasia esculenta (L.) Schott: Pharmacognostic and pharmacological review. Phytopathology 2020, 9, 1382–1386. [Google Scholar]
- Rieger, M.A.; Schroeder, T. Hematopoiesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008250. [Google Scholar] [CrossRef] [PubMed]
- Alexander, W.S. Cytokines in hematopoiesis. Int. Rev. Immunol. 1998, 16, 651–682. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D. Hematopoietic cytokines. Blood 2008, 111, 485–491. [Google Scholar] [CrossRef] [Green Version]
- de Rezende, M.M.; Ng-Blichfeldt, J.-P.; Justo, G.Z.; Paredes-Gamero, E.J.; Gosens, R. Divergent effects of Wnt5b on IL-3- and GM-CSF-induced myeloid differentiation. Cell. Signal. 2020, 67, 109507. [Google Scholar] [CrossRef]
- Akashi, K.; Traver, D.; Miyamoto, T.; Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000, 404, 193–197. [Google Scholar] [CrossRef]
- Kondo, M.; Weissman, I.L.; Akashi, K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 1997, 91, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.Y.; Wang, J.-X.; Parisini, E.; Dascher, C.C.; Nigrovic, P.A. Ly6 family proteins in neutrophil biology. J. Leukoc. Biol. 2013, 94, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Hestdal, K.; Ruscetti, F.; Ihle, J.; Jacobsen, S.; Dubois, C.; Kopp, W.; Longo, D.; Keller, J. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J. Immunol. 1991, 147, 22–28. [Google Scholar]
- Mendelson, A.; Frenette, P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014, 20, 833. [Google Scholar] [CrossRef] [Green Version]
- Vial, T.; Descotes, J. Clinical toxicity of cytokines used as haemopoietic growth factors. Drug Saf. 1995, 13, 371–406. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Zhang, Y. Risks of Venous Thromboembolism, Stroke, Heart Disease, and Myelodysplastic Syndrome Associated With Hematopoietic Growth Factors in a Large Population-Based Cohort of Patients With Colorectal Cancer. Clin. Colorectal Cancer 2015, 14, 21–31. [Google Scholar] [CrossRef]
- Aurrand-Lions, M.; Mancini, S.J.C. Murine Bone Marrow Niches from Hematopoietic Stem Cells to B Cells. Int. J. Mol. Sci. 2018, 19, 2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haseeb, M.; Anwar, A.; Choi, S. Molecular Interactions Between Innate and Adaptive Immune Cells in Chronic Lymphocytic Leukemia and Their Therapeutic Implications. Front. Immunol. 2018, 9, 2720. [Google Scholar] [CrossRef]
- Alves, N.L.; van Leeuwen, E.M.; Derks, I.A.; van Lier, R.A. Differential regulation of human IL-7 receptor alpha expression by IL-7 and TCR signaling. J. Immunol. 2008, 180, 5201–5210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudo, T.; Nishikawa, S.-I.; Ohno, N.; Akiyama, N.; Tamakoshi, M.; Yoshida, H. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl. Acad. Sci. USA 1993, 90, 9125–9129. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.C.; Reitzenstein, J.E.; Liu, J.; Jadus, M.R. The anti-cancer effects of poi (Colocasia esculenta) on colonic adenocarcinoma cells In vitro. Phytother. Res. 2005, 19, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Merling, A.; Karsten, U.; Schwartz-Albiez, R. The fucosylated histo-blood group antigens H type 2 (blood group O, CD173) and Lewis Y (CD174) are expressed on CD34+ hematopoietic progenitors but absent on mature lymphocytes. Glycobiology 2001, 11, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Dettke, M.; Palfi, G.; Loibner, H. Activation-dependent expression of the blood group-related lewis Y antigen on peripheral blood granulocytes. J. Leukoc. Biol. 2000, 68, 511–514. [Google Scholar] [PubMed]
- Stella, C.C.; Cazzola, M.; De Fabritiis, P.; De Vincentiis, A.; Gianni, A.M.; Lanza, F.; Lauria, F.; Lemoli, R.M.; Tarella, C.; Zanon, P. CD34-positive cells: Biology and clinical relevance. Haematologica 1995, 80, 367. [Google Scholar]
- Hermiston, M.L.; Xu, Z.; Weiss, A. CD45: A critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 2003, 21, 107–137. [Google Scholar] [CrossRef]



| Experimental Condition | Tarin Stimulus (µg/mL) | % Occupied Area/Field 1 | Number of Clusters and Colonies/Field | ||
|---|---|---|---|---|---|
| Day 3 | Day 12 | Day 3 | Day 12 | ||
| Control | - | 51.2 ± 5.2 | 31.6 ± 3.5 | 2.5 ± 2.4 | 0.0 ± 0.0 |
| Tarin | 6.0 | 68.0 ± 2.3 * | 26.8 ± 3.7 | 4.5 ± 2.1 | 1.8 ± 0.1 |
| 12.0 | 63.9 ± 2.7 * | 44.1 ± 2.9 * | 13.8 ± 2.2 ** | 3.3 ± 1.0 | |
| 25.0 | 64.4 ± 2.3 * | 84.2 ± 3.2 * | 17.8 ± 3.3 * | 17.5 ± 3.4 * | |
| 50.0 | 64.1 ± 3.1* | 85.2 ± 3.2 * | 28.5 ± 4.2 * | 19.8 ± 5.0 * | |
| 100.0 | 78.4 ± 2.6 * | 91.4 ± 3.7 * | 29.5 ± 3.9 * | 18.3 ± 2.6 * | |
| Gate | Cell Phenotype | Control (%) | Tarin (%) |
|---|---|---|---|
| A | GR-1+ | 8.8 ± 2.5 | 23.3 ± 4.3 *** |
| B | CD3+ | 19.6 ± 1.4 | 48.3 ± 3.3 ** |
| CD19+ | 32.5 ± 2.5 | 21.1 ± 2.5 ** |
| Stimulus | Gate B (%) |
|---|---|
| Control | 27.2 ± 2.1 a |
| Tarin | 9.7 ± 0.7 * b |
| cmIL-3 | 21.1 ± 1.3 c |
| cmIL-3 + tarin | 11.4 ± 0.7 # b |
| cmGM-CSF | 22.7 ± 0.3 c |
| cmGM-CSF + tarin | 26.5 ± 1.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattos, E.B.d.A.; Pereira, P.R.; Mérida, L.A.D.; Corrêa, A.C.N.T.F.; Freire, M.P.V.; Paschoalin, V.M.F.; Teixeira, G.A.P.B.; Pinho, M.d.F.B.; Verícimo, M.A. Taro Lectin Can Act as a Cytokine-Mimetic Compound, Stimulating Myeloid and T Lymphocyte Lineages and Protecting Progenitors in Murine Bone Marrow. Pharmaceutics 2021, 13, 350. https://doi.org/10.3390/pharmaceutics13030350
Mattos EBdA, Pereira PR, Mérida LAD, Corrêa ACNTF, Freire MPV, Paschoalin VMF, Teixeira GAPB, Pinho MdFB, Verícimo MA. Taro Lectin Can Act as a Cytokine-Mimetic Compound, Stimulating Myeloid and T Lymphocyte Lineages and Protecting Progenitors in Murine Bone Marrow. Pharmaceutics. 2021; 13(3):350. https://doi.org/10.3390/pharmaceutics13030350
Chicago/Turabian StyleMattos, Erika Bertozzi de Aquino, Patricia Ribeiro Pereira, Lyris Anunciata Demétrio Mérida, Anna Carolina Nitzsche Teixeira Fernandes Corrêa, Maria Paula Vigna Freire, Vania Margaret Flosi Paschoalin, Gerlinde Agate Platais Brasil Teixeira, Maria de Fátima Brandão Pinho, and Maurício Afonso Verícimo. 2021. "Taro Lectin Can Act as a Cytokine-Mimetic Compound, Stimulating Myeloid and T Lymphocyte Lineages and Protecting Progenitors in Murine Bone Marrow" Pharmaceutics 13, no. 3: 350. https://doi.org/10.3390/pharmaceutics13030350
APA StyleMattos, E. B. d. A., Pereira, P. R., Mérida, L. A. D., Corrêa, A. C. N. T. F., Freire, M. P. V., Paschoalin, V. M. F., Teixeira, G. A. P. B., Pinho, M. d. F. B., & Verícimo, M. A. (2021). Taro Lectin Can Act as a Cytokine-Mimetic Compound, Stimulating Myeloid and T Lymphocyte Lineages and Protecting Progenitors in Murine Bone Marrow. Pharmaceutics, 13(3), 350. https://doi.org/10.3390/pharmaceutics13030350