3.1. Synthesis and Characterization of β-Cyclodextrin-Based Nanosponges
Different synthesis routes have been reported for βCDNS formation, and they use ultrasonic baths; heating plates; solvents, such as ethanol or acetone for the washing stages; and even different molar ratios of βCD and DPC [
7,
10]. For this reason, different methodologies were evaluated to optimize the synthesis of βCDNS, eliminate byproducts, and increase yield. For the ultrasonic bath (A) and heating plate (B) methods, the use of acetone and a 1:4 molar ratio showed yields greater than 60%, as shown in
Figure A1 (
Appendix A). Considering the reproducibility of the synthesis and the lower amount of generated byproducts exhibited by method B relative to method A, method B with a heating plate was selected.
βCDNS formation was confirmed using
1H-NMR. The technique allowed us to compare the chemical shifts of the signals for βCD protons in βCDNS and in native βCD.
Figure 1 shows the spectra of (A) βCD, (B) DPC, and (C) βCDNS with a scheme showing the proton assignments for βCD and DPC.
Table 1 shows the proton assignment for βCD and their respective chemical shifts and integrations in the
1H-NMR spectra. The shifts of the signals are due to the change in the chemical environment of the βCD matrices when they are linked to form βCDNS. Notably, the greatest changes were observed in the integration delta (Δ∫) of the hydroxyl groups, because they react with DPC to form linkers between βCD matrices, strongly suggesting βCDNS formation.
βCDNS formation was also characterized using IR vibrational spectroscopy. Commonly, this study focuses on comparing the signals of native βCD and βCD forming nanosponges and recognizing the vibration signal of the carbonyl group, which is an indicator of βCD crosslinking.
Figure 2A shows the IR spectra of (A) βCD, (B) DPC, and (C) βCDNS. Characteristic peaks of βCD are observed at 3363 cm
−1 (O-H alcohol stretching), 2924 cm
−1 (C-H stretching), 1417 cm
−1, 1368 cm
−1, 1157 cm
−1 (O-H bending), 1080 cm
−1, and 1029 cm
−1 (C-O stretching). These data are consistent with literature data [
63,
64,
65]. For βCDNS, the characteristic peaks are located mostly in the same regions observed for βCD, but with shifts or variations in intensity due to changes in the chemical environment. These were observed at 3366 cm
−1 (O-H alcohol stretching), 2928 cm
−1 (C-H stretching), 1645 cm
−1 (C=O stretching), 1367, 1234, and 1155 cm
−1 (O-H bending), and 1079 cm
−1 and 1030 cm
−1 (C-O stretching). Notably, the appearance of peaks at 1783, 1715, and 1235 cm
−1 derived from signals present in DPC confirm the crosslinking of βCD forming nanosponges. The peak at 1760 cm
−1 (C=O stretching) of DPC is masked by a peak in the βCDNS spectrum.
Thermogravimetry was performed to analyze and confirm the formation of βCDNS, differentiating it from its precursors through changes in their thermal decomposition, as is typically observed in the synthesis of polymeric materials [
66].
Figure 2B shows thermograms of (A) βCD, (B) DPC, and (C) βCDNS. The loss of hydration water was observed in the first decomposition at temperatures up to 100 °C, with the percentage of mass loss being 11.5% for βCD and 2.7% for βCDNS of the total mass samples. Decomposition of 100% of the mass of DPC was observed in the range 130 to 250 °C. A second range of decomposition in βCD was observed between 300 and 350 °C, corresponding to a loss of 71% of the sample mass. For βCDNS, this second range was between 210 and 350 °C, consuming 70% of the total mass. The decrease in the temperature at the beginning of thermal degradation suggests that DPC, a crosslinker molecule, binds to the primary OH groups of βCDs, forming the nanopolymer through carbonyl groups. Changes in the peaks of the TGA curves (see
Figure A2 in
Appendix A) from 337 °C (βCD) to 327 (βCDNS) are typically observed in the formation of polymeric materials due to changes in chemical structure [
67,
68,
69]. Finally, the oxidation interval for βCD ranged from 350 to 700 °C, encompassing 17.5% of the mass. However, βCDNS oxidation ranges from 350 to 580 °C, encompassing 27.3% of the mass. This also suggests modifications in the reactive structure of the polymer relative to native βCD.
To explain the change in the beginning of the range of thermal degradation for βCDNS, the average between the beginning temperatures for βCD and DPC, which were 300 and 130 °C, respectively, was evaluated. The calculated average temperature was 215 °C, which coincided with the value of the beginning of thermal degradation observed in the βCDNS thermogram, fulfilling the “eutectic mixture” criterion [
70]. In addition, the high value of the degradation interval for βCDNS supports its thermal stability.
To obtain information on the morphology and size of βCDNS, the material was characterized using electron microscopy techniques and DLS.
Figure 3 shows micrographs obtained by FE-SEM of native βCD (
Figure 3A) and βCDNS (
Figure 3B), directly revealing the morphological differences between both. βCD has irregular crystalline structures, while βCDNS has a characteristic porous appearance. TEM images were obtained to determine the average diameter of βCDNS, which were previously dispersed by sonication.
Figure 3C,D shows the βCDNS and the resulting histogram, respectively. The average diameter, obtained from the count of more than 450 nanoparticles seen in various TEM images, was 146 ± 54 nm (see more images in
Figure A3 in
Appendix A). The staining of the βCDNS sample revealed some βCD crystals, which was verified by obtaining TEM images of native βCD with the same dispersion and staining protocol described for βCDNS (see
Figure A3 in
Appendix A). In addition, a hydrodynamic diameter of 133.9 ± 66.9 nm was found for βCDNS using DLS. These size data are concordant and strongly suggest the nanometric dimensions of the system studied (see more details in
Appendix C).
3.2. Loading of β-Cyclodextrin-Based Nanosponges with Drugs
The βCDNS obtained was loaded with two drugs separately, forming the βCDNS–PhEA and βCDNS–AT systems. Once each supramolecular complex was formed in the solubilized phase of the aqueous solution, the effective inclusion of the drugs and the stoichiometric relationship of both systems were analyzed using 1H-NMR.
Figure 4 shows the spectra of βCDNS–PhEA (A) and βCDNS–AT (B) with their molecular structures and proton assignments for the respective drug. The loading of PhEA to form the βCDNS–PhEA system (A) and the loading of AT to form the βCDNS–AT system (B) were confirmed with the respective assignments of protons in the molecular structures of PhEA and AT (see full spectra,
Figure A4 and
Figure A5, in
Appendix B).
Table 2 and
Table 3 show the chemical shifts and integrals recorded for the protons of βCDNS and of the PhEA and AT drugs resulting from the inclusion process.
For the βCDNS–PhEA system,
Table 2, the largest chemical shifts for βCDNS were observed for the internal protons H3 and H5 and the hydroxyl groups OH2 and OH3, probably due to preferential inclusion in the widest zone of the βCD cavity. In addition, chemical shifts for all the βCDNS protons were observed, mainly towards lower fields, which demonstrates the effective loading of PhEA within βCD cavities and in the multiple interstitial spaces of the interstitial βCDNS produced by crosslinking. Analyzing the chemical shifts of the PhEA protons, a change in the chemical environment due to inclusion was also evidenced, consistent with that reported in the literature [
25,
71].
For the βCDNS–AT system,
Table 3, chemical shifts were observed in all the βCDNS protons oriented towards the interior and exterior of the cavity due to the change in the chemical environment of βCDNS resulting from AT loading. This finding shows that the inclusion of the drug occurs in βCD cavities and between the formed interstitial spaces. Chemical shifts towards higher fields were observed in the protons NH
2b, Hb’/f’, and Hc’/e’ of AT, which demonstrates the electronic shielding effect of the drug due to its inclusion in the nanosponges, in accordance with that reported in the literature [
26].
Notably, the integration of the βCDNS protons and the protons of each drug in their respective
1H-NMR spectra,
Table 2 and
Table 3, showed a stoichiometric βCD:drug ratio of 1:8 in both systems, which is an amount of drug eight times greater than those reported for βCD–PhEA [
25] and βCD–AT [
26], each of which exhibits a 1:1 stoichiometry. This amount is equivalent to 0.9 mg of PhEA loading per 1 mg of βCD unit in βCDNS, and on the other hand, to 1.5 mg of AT loading per 1 mg of βCD unit in βCDNS. Applying Equation (1) [
58] (
Section 2, Material and Methods), the loading capacity in βCDNS is 90% for PhEA and 150% for AT, which is higher than the loading capacity of 11% for PhEA and 19% for AT in βCD native, according to reported data [
25,
26]. These results show that the drug loading of the βCDNS formed is higher than that of native βCD and that βCDNS could be used as a more efficient drug carrier than native βCD (see the details in the
Appendix B).
The loading of drugs into βCDNS was also analyzed by FT-IR spectroscopy by comparing peaks for vibrations before and after the inclusion process.
Figure 5 shows the spectra of (A) PhEA, (B) βCDNS-PhEA, (C) AT, and (D) βCDNS–AT.
In the vibrational analysis of the βCDNS–PhEA system, the βCDNS peaks at 3570 cm−1 and 3170 cm−1 corresponding to O-H alcohol stretching and N-H primary amine asymmetric and symmetric stretching, respectively, were identified. The peaks at 2926 cm−1 corresponding to C-H stretching, at 1642 cm−1 corresponding to C=O stretching, at 1333 cm−1 and 1157 cm−1 corresponding to O-H bending, and at 1081 cm−1 and 1029 cm−1 corresponding to C-O stretching were also identified. These vibrations remain unchanged in comparison to those of the βCDNS spectrum without loaded drugs. The peak from PhEA found for the βCDNS–PhEA system corresponding to N-H symmetric stretching was observed at 2950 cm−1, while the peak at 745 cm−1 corresponding to C-H aromatics was masked due to the inclusion process.
In the case of the βCDNS–AT system, decreases in the intensity of some peaks with respect to those of βCDNS were observed. However, the characteristic peaks were located in the same regions of the spectra. O-H alcohol stretching, and N-H primary amine asymmetric and symmetric stretching vibrations were observed at 3170 cm−1 and 3570 cm−1, respectively. C-H stretching appeared at 2924 cm−1, C=O stretching at 1637 cm−1, O-H group bending at 1384 cm−1 and 1157 cm−1, and finally, C-O stretching appeared at 1079 cm−1 and 1029 cm−1. The characteristic peaks of AT at 1476 cm−1, corresponding to C=C aromatics, and at 3438 cm−1, corresponding to N-H aromatic stretching, were masked in βCDNS–AT due to the inclusion in βCDNS.
The changes in the intensity and definition of the βCDNS peaks observed in the IR spectra suggested a change in their conformations due to drug loading, which was also corroborated by DLS and TEM. The hydrodynamic diameters of βCDNS–PhEA and βCDNS–AT were 270.5 ± 48.0 nm and 335.5 ± 150.5 nm, respectively, observing an increase in the size of both systems with respect to βCDNS (see more details in
Appendix C).
Figure 6 shows TEM images of βCDNS loaded with PhEA (A–E) and AT (F–I). Changes in the shapes of the systems with respect to that of βCDNS were also observed; in addition, the average diameter calculated using TEM images of these systems increased to 252 ± 39 nm with respect to βCDNS. The loading of the drugs PhEA and AT could promote a process of association and intermolecular interactions between different βCDNS. This would explain the increase in size observed using TEM and DLS.
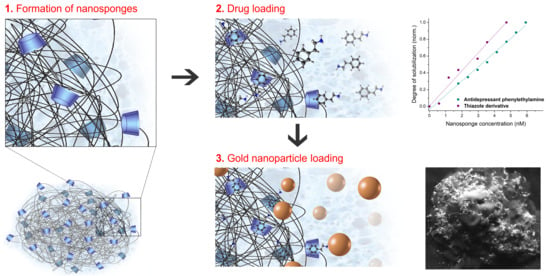
The degree of solubilization indicates the tendency to increase the aqueous solubility of the drugs due to the action of βCDNS, while the complexation efficiency corresponds to the concentration of drug included versus the concentration of drug initially used in the process. This is directly related to the effectiveness of βCDNS and intermolecular interactions to keep drugs entrapped in the complex. The degree of solubilization of the drugs, the
Ka, and the complexation efficiency for the βCDNS–PhEA and βCDNS–AT systems were calculated using phase solubility studies (Equations (2) and (3),
Section 2, Material and Methods) [
59] and are shown in
Table 4. Additionally, they were compared with the results obtained for the complexation of PhEA and AT using native βCD [
25,
26].
An increase in the aqueous solubility of PhEA and AT using βCDNS was observed, when they were compared to the solubility of free drugs (see
Figure A7 and
Figure A8,
Appendix B). Notably, the degree of solubilization achieved by the presence of βCDNS was more than 1.3 times higher for PhEA and 5 times higher for AT than with native βCD. This is especially relevant in therapy since drugs to be pharmacologically active must be soluble in water. The
Ka values are 1318 M
−1 and 484 M
−1 for the βCDNS–PhEA and βCDNS–AT systems, respectively. These results indicate that the interactions that allow inclusion are strong, forming two highly stable systems over time due to the incorporation of βCDNS. The complexation efficiency values obtained for both systems show that the complexation using βCDNS is optimal, being the same for PhEA in native βCD and seven times greater for AT in native βCD. The above findings are in accordance with the previous discussion given by stoichiometry studies and loading capacity calculated using NMR (more details in the
Appendix B).
In general, the K
a values of the βCD complexes vary between 50 and 2000 M
−1. Lower values at 50 M
−1 indicate a limitation in the pharmaceutical formulation since they have low stability and do not release the drug at its site of action [
25,
72,
73,
74]. On the other hand, K
a values greater than 2000 M
−1 also present limitations, such as poor pharmacokinetics, since the drug release rates can be affected [
72,
73]. This is why the use of a strategy for the controlled release of the drugs included in βCDNS becomes relevant. AuNPs can release absorbed energy in the form of heat and can release molecules near their surface as a result of the photothermal effect [
28,
75,
76]. This was demonstrated for a drug in AuNP- and βCD-based systems using laser irradiation [
25,
47]. In this sense, the incorporation of AuNPs into the two systems could, in addition to acting as a therapeutic agent, promote the controlled release of the drugs.
3.3. Synthesis and Immobilization of Gold Nanoparticles on Drug-Loaded β-Cyclodextrin-Based Nanosponges
Once the βCDNS–drug systems were obtained, the interactions with colloidal AuNPs were studied to load another therapeutic agent and form the βCDNS–PhEA–AuNP and βCDNS–AT–AuNP systems. AuNPs were synthesized following the Turkevich method at pH 5.5. These AuNPs were then stabilized at pH 8.8 to facilitate their immobilization on drug-loaded βCDNS.
Figure 7A shows the absorbance spectra of AuNPs at pH 5.5 and 8.8, and
Figure 7B shows a representative TEM micrograph of spherical AuNPs with an average diameter of 18 ± 4 nm (see histogram in
Figure A9,
Appendix C) AuNPs with diameters between 4 and 100 nm do not present cytotoxic effects [
77], which would allow possible drug delivery applications.
Figure 7C,D shows the UV-Vis spectra of the βCDNS–PhEA–AuNP and βCD–AT–AuNP systems, respectively, in addition to those of the initial AuNP solution and the supernatant resulting from the functionalization of each mixture. The recorded plasmon bands demonstrate a preferential interaction of AuNPs with βCDNS–drug, with an immobilization of 85%, maintaining the main characteristics of the plasmon band and indicating that AuNPs remain stable in both systems.
Table 5 shows the intensities and the maximum wavelengths from the absorbance spectra. In addition, the hydrodynamic diameter and surface charge of βCDNS–PhEA–AuNP and βCDNS–AT–AuNP in aqueous solution are shown. These analyses represent the behavior of AuNPs in the different systems, because Au is highly efficient to absorb and scatter light, being superior to the organic material present.
A shift in the wavelength of the maximum absorbances with respect to those for the as-synthesized AuNPs occurred for both systems due to the interparticle coupling caused by the increased proximity between these nanostructures when immobilized; in turn, the permanence of the plasmon bands was evidence of the stability achieved and that the aggregation of AuNPs did not occur. In turn, increases in hydrodynamic diameters from 33.9 ± 13.2 nm for AuNPs with citrate to 51.2 ± 24.7 nm for AuNPs in the βCDNS–PhEA–AuNP system and up to 114.0 ± 42.2 nm for AuNPs in the βCDNS–AT system were observed due to the proximity between the immobilized AuNPs and the presence of βCDNS–drug complexes. Furthermore, this behavior was consistent with the increase in size of the βCDNS when they were loaded with the drugs. The reported partial and dynamic inclusion of AT in βCD could explain the greater hydrodynamic diameter of the AuNPs on βCDNS–AT with respect to βCDNS–PhEA. The two functional groups, NH
2 and SH, of AT are exposed [
26], facilitating its interaction with AuNPs, while PhEA only has one NH
2 group that is completely included within βCD [
25,
71].
The registered surface charge of the AuNPs was −51.4 ± 7.9 mV due to the stabilizing citrate ions, which changed to −33.0 ± 5.3 mV and −38.4 ± 6.9 mV for AuNPs in the βCDNS–PhEA–AuNP and in the βCDNS–AT–AuNP systems, respectively, due to the replacement of a fraction of citrate molecules by neutral supramolecular complexes. As a control, a drug-free βCDNS solution was subjected to the same mixing protocol with colloidal AuNPs, confirming through different characterization techniques that the interaction between βCDNS and AuNPs does not occur (see the details in the
Appendix C).
Figure 8A,B shows SEM micrographs of the βCDNS–PhEA–AuNP (A) and βCDNS–AT–AuNP (B) systems, respectively. The images clearly show the AuNPs immobilized on the βCDNS–drug supramolecular complexes. In addition, an irregular morphology was observed, probably due to the process of functionalization of βCDNS, as suggested by the TEM images (
Figure 6).
Various characterization techniques and direct observation using electron microscopy confirmed the simultaneous loading of βCDNS with two therapeutic agents, drugs and AuNPs, forming the βCDNS–PhEA–AuNP and βCDNS–AT–AuNP systems. If properly designed, that is, by establishing parameters for the colloidal stability, concentration, surface charge, and size, among others, βCDNS and AuNPs could be considered nontoxic and used in therapy without generating adverse effects in the organism. In this sense, in the design and formation of these two new systems, the established parameters were realized.