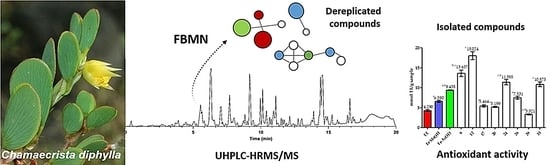
Constituents of Chamaecrista diphylla (L.) Greene Leaves with Potent Antioxidant Capacity: A Feature-Based Molecular Network Dereplication Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Sample Preparation
2.3. Isolation of Compounds
2.4. Determination of the Total Phenols
2.5. Determination of Total Flavonoids
2.6. Scavenging Antioxidant Activity
2.7. Calculation of Bond Dissociation Enthalpies O–H
2.8. UHPLC-MS/MS Analysis
2.9. Metabolite Characterization Workflow
3. Results
3.1. Antioxidant Activity Assessment of Chamaecrista diphylla Extract and Enriched Fractions
3.2. Dereplication and Isolation of Compounds from Chamaecrista diphylla
3.3. Antioxidant Activity Evaluation of the Isolated Compounds
3.4. Bond Dissociation Enthalpy (BDE) Calculations for Selected Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shokolenko, I.; Venediktova, N.; Bochkareva, A.; Wilson, G.L.; Alexeyev, M.F. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009, 37, 2539–2548. [Google Scholar] [CrossRef] [Green Version]
- Poljsak, B.; Milisav, I. The neglected significance of “antioxidative stress”. Oxid. Med. Cell. Longev. 2012, 2012, 480895. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- França, B.K.; Melo Alves, M.R.; Silveira Souto, F.M.; Tiziane, L.; Freire Boaventura, R.; Guimarães, A.; Alves, A., Jr. Peroxidação lipídica e obesidade: Métodos para aferição do estresse oxidativo em obesos. GE J. Port. Gastr. 2013, 20, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Kannan, K. Quantitative identification of and exposure to synthetic phenolic antioxidants, including butylated hydroxytoluene, in urine. Environ. Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.; Zhang, W. A review of antioxidant peptides derived from meat muscle and by-products. Antioxid. Redox Signal 2016, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Dimitrios, B. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Boutin, J.A.; Jockers, R. Melatonin controversies, an update. J. Pineal Res. 2020, e12702. [Google Scholar] [CrossRef]
- Plants, U.T. Useful Tropical Plants Database. Available online: http://tropical.theferns.info/ (accessed on 5 January 2021).
- Lewis, G.P.; Schrire, B.; Mackinder, B.; Lock, M. Legumes of the World; Royal Botanic Gardens, Kew: London, UK, 2005; ISBN 9781900347808. [Google Scholar]
- Zemmouri, H.; Ammar, S.; Boumendjel, A.; Messarah, M.; El Feki, A.; Bouaziz, M. Chemical composition and antioxidant activity of Borago officinalis L. leaf extract growing in Algeria. Arab. J. Chem. 2019, 12, 1954–1963. [Google Scholar] [CrossRef]
- New York Botanical Garden. Memoirs of the New York Botanical Garden; New York Botanical Garden: New York, NY, USA, 1900; ISBN 9780893272418. [Google Scholar]
- Silva, W.L.; Rocha, A.E.; Santos, J.U.M. Dos Leguminosae em savanas do estuário amazônico brasileiro. Rodriguésia 2014, 65, 329–353. [Google Scholar] [CrossRef] [Green Version]
- de Souza, L.A.G. Guia da biodiversidade de fabaceae do Alto Rio Negro; Projeto Fronteiras: Manaus, Brazil, 2012. [Google Scholar]
- Reis, J.D.E.; Gomes, P.W.P.; Muribeca, A.D.J.B.; de Castro, M.N.R. Quantification of phenolic derivatives and antioxidant activity of the leaves of Chamaecrista diphylla (L.) Greene (Fabaceae). Sci. Plena 2020, 16. [Google Scholar] [CrossRef] [Green Version]
- Lopes, K.; Oliveira, J.; Sousa-Junior, F.J.C.; Santos, T.d.F.; Andrade, D.; Andrade, S.L.; Pereira, W.L.; Gomes, P.W.P.; Monteiro, M.C.; Silva, E.; et al. Chemical Composition, Toxicity, Antinociceptive, and Anti-Inflammatory Activity of Dry Aqueous Extract of Varronia multispicata (Cham.) Borhidi (Cordiaceae) Leaves. Front. Pharmacol. 2019, 10, 1376. [Google Scholar] [CrossRef]
- Fujishima, M.A.T.; Sá, D.M.C.; Lima, C.M.d.S.; Bittencourt, J.A.H.M.; Pereira, W.L.A.; Muribeca, A.d.J.B.; E Silva, C.Y.Y.; da Silva, M.N.; de Sousa, F.F.O.; Dos Santos, C.B.R.; et al. Chemical profiling of Curatella americana Linn leaves by UPLC-HRMS and its wound healing activity in mice. PLoS ONE 2020, 15, e0225514. [Google Scholar] [CrossRef]
- Borges, L.d.C.; Negrão-Neto, R.; Pamplona, S.; Fernandes, L.; Barros, M.; Fontes-Júnior, E.; Maia, C.; E Silva, C.Y.Y.; Silva, M.N.D. Anti-Inflammatory and Antinociceptive Studies of Hydroalcoholic Extract from the Leaves of Phyllanthus brasiliensis (Aubl.) Poir. and Isolation of 5-O-β-d-Glucopyranosyljusticidin B and Six Other Lignans. Molecules 2018, 23, 941. [Google Scholar] [CrossRef] [Green Version]
- Joseph, R.C.; Silva da Fonseca Diniz, M.; Magno do Nascimento, V.; Barbosa Muribeca, A.d.J.; Costa Santiago, J.C.; da Cunha Borges, L.; da Costa Sá, P.R.; Portal Gomes, P.W.; da Silva Cardoso, J.C.; de Castro Rocha, M.N.; et al. Secure and Sustainable Sourcing of Plant Tissues for the Exhaustive Exploration of Their Chemodiversity. Molecules 2020, 25, 5992. [Google Scholar] [CrossRef]
- Lautié, E.; Russo, O.; Ducrot, P.; Boutin, J.A. Unraveling Plant Natural Chemical Diversity for Drug Discovery Purposes. Front. Pharmacol. 2020, 11, 397. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Delcour, J.A.; Varebeke, D.J. De a new colourimetric assay for flavanoids in pilsner beers. J. Inst. Brew. 1985, 91, 37–40. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.; Souza, J.N.S.; Rogez, H.; Rees, J.F.; Larondelle, Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 2007, 101, 1012–1018. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [Green Version]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Quirós-Guerrero, L.; Albertazzi, F.; Araya-Valverde, E.; Romero, R.M.; Villalobos, H.; Poveda, L.; Chavarría, M.; Tamayo-Castillo, G. Phenolic variation among Chamaecrista nictitans subspecies and varieties revealed through UPLC-ESI(-)-MS/MS chemical fingerprinting. Metabolomics 2019, 15, 14. [Google Scholar] [CrossRef]
- Mateos-Martín, M.L.; Fuguet, E.; Jiménez-Ardón, A.; Herrero-Uribe, L.; Tamayo-Castillo, G.; Torres, J.L. Identification of polyphenols from antiviral Chamaecrista nictitans extract using high-resolution LC-ESI-MS/MS. Anal. Bioanal. Chem. 2014, 406, 5501–5506. [Google Scholar] [CrossRef]
- Tangavelou, A.C.; Viswanathan, M.B.; Balakrishna, K.; Patra, A. Phytochemical Analysis in the Leaves of Chamaecrista nigricans (Leguminosae). Pharm. Anal. Acta 2018, 9. [Google Scholar] [CrossRef]
- Sebei, K.; Sbissi, I.; Souhir, A.; Herchi, W.; Ssakouhi, F.; Boukhchina, S. Phylogenetic identification, phytochemical analysis and antioxidant activity of Chamaecrista absus var. absus seeds. J. Plant Biol. Res. 2014, 3, 1–11. [Google Scholar]
- Barba, B.; Díaz, J.G.; Herz, W. Cassanes and anthraquinones from Chamaecrista greggii. Phytochemistry 1994, 37, 837–845. [Google Scholar] [CrossRef]
- Holman, J.D.; Tabb, D.L.; Mallick, P. Employing ProteoWizard to Convert Raw Mass Spectrometry Data. Curr. Protoc. Bioinform. 2014, 46, 1–9. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Allard, P.M.; Péresse, T.; Bisson, J.; Gindro, K.; Marcourt, L.; Pham, V.C.; Roussi, F.; Litaudon, M.; Wolfender, J.L. Integration of Molecular Networking and In-Silico MS/MS Fragmentation for Natural Products Dereplication. Anal. Chem. 2016, 88, 3317–3323. [Google Scholar] [CrossRef]
- Rutz, A.; Dounoue-Kubo, M.; Ollivier, S.; Bisson, J.; Bagheri, M.; Saesong, T.; Ebrahimi, S.N.; Ingkaninan, K.; Wolfender, J.L.; Allard, P.M. Taxonomically Informed Scoring Enhances Confidence in Natural Products Annotation. Front. Plant Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Ma, Y.L.; Vedernikova, I.; van den Heuvel, H.; Claeys, M. Internal glucose residue loss in protonated O-diglycosyl flavonoids upon low-energy collision-induced dissociation. J. Am. Soc. Mass Spectrom. 2000, 11, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.A.M.; Yariwake, J.H.; McCullagh, M. Distinction of the C-glycosylflavone isomer pairs orientin/isoorientin and vitexin/isovitexin using HPLC-MS exact mass measurement and in-source CID. Phytochem. Anal. 2005, 16, 295–301. [Google Scholar] [CrossRef]
- Salpin, J.-Y.; Tortajada, J. Structural characterization of hexoses and pentoses using lead cationization. An electrospray ionization and tandem mass spectrometric study. J. Mass Spectrom. 2002, 37, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Cuyckens, F.; Ma, Y.L.; Pocsfalvi, G.; Claeysi, M. Tandem mass spectral strategies for the structural characterization of flavonoid glycosides. Analusis 2000, 28, 888–895. [Google Scholar] [CrossRef] [Green Version]
- Kováčik, V.; Hirsch, J.; Kováč, P.; Heerma, W.; Thomas-Oates, J.; Haverkamp, J. Oligosaccharide characterization using collision-induced dissociation fast atom bombardment mass spectrometry: Evidence for internal monosaccharide residue loss. J. Mass Spectrom. 1995, 30, 949–958. [Google Scholar] [CrossRef]
- Brüll, L.P.; Heerma, W.; Thomas-Oates, J.; Haverkamp, J.; Kovácik, V.; Kovác, P. Loss of internal 1 → 6 substituted monosaccharide residues from underivatized and per-O-methylated trisaccharides. J. Am. Soc. Mass Spectrom. 1997, 8, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Brüll, L.P.; Kovácik, V.; Thomas-Oates, J.E.; Heerma, W.; Haverkamp, J. Sodium-cationized oligosaccharides do not appear to undergo “internal residue loss” rearrangement processes on tandem mass spectrometry. Rapid Commun. Mass Spectrom. 1998, 12, 1520–1532. [Google Scholar] [CrossRef]
- Azizah, M.; Pripdeevech, P.; Thongkongkaew, T.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. UHPLC-ESI-QTOF-MS/MS-Based Molecular Networking Guided Isolation and Dereplication of Antibacterial and Antifungal Constituents of Ventilago denticulata. Antibiotics 2020, 9, 606. [Google Scholar] [CrossRef]
- Smyth, W.F.; Morgan, J.L.; O’Kane, E. The characterisation of coumarins from selected structural classes by electrospray ionisation quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 15, 1308–1314. [Google Scholar] [CrossRef]
- Königs, P.; Rinker, B.; Maus, L.; Nieger, M.; Rheinheimer, J.; Waldvogel, S.R. Structural revision and synthesis of altechromone A. J. Nat. Prod. 2010, 73, 2064–2066. [Google Scholar] [CrossRef]
- Strehmel, N.; Böttcher, C.; Schmidt, S.; Scheel, D. Profiling of secondary metabolites in root exudates of Arabidopsis thaliana. Phytochemistry 2014, 108, 35–46. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Factors influencing the antioxidant activity determined by the ABTS.+ radical cation assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. The importance of solvent type in estimating antioxidant properties of phenolic compounds by ABTS assay. Eur. Food Res. Technol. 2013, 236, 1099–1105. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.M.; Machado, N.F.L.; Marques, M.P.M. Dietary chromones as antioxidant agents—the structural variable. Food Funct. 2011, 2, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Csepanyi, E.; Szabados-Furjesi, P.; Kiss-Szikszai, A.; Frensemeier, L.M.; Karst, U.; Lekli, I.; Haines, D.D.; Tosaki, A.; Bak, I. Antioxidant Properties and Oxidative Transformation of Different Chromone Derivatives. Molecules 2017, 22, 588. [Google Scholar] [CrossRef] [Green Version]
- Kładna, A.; Berczyński, P.; Piechowska, T.; Kruk, I.; Aboul-Enein, H.Y.; Ceylan-Unlusoy, M.; Verspohl, E.J.; Ertan, R. Studies on the antioxidant activities of some new chromone compounds. Luminescence 2014, 29, 846–853. [Google Scholar] [CrossRef]
- Phosrithong, N.; Samee, W.; Nunthanavanit, P.; Ungwitayatorn, J. In vitro antioxidant activity study of novel chromone derivatives. Chem. Biol. Drug Des. 2012, 79, 981–989. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Ellison, A.M. Princípios de Estatística em Ecologia; Artmed Editora: Porto Alegre, RS, Brazil, 2016; ISBN 9788536324692. [Google Scholar]
- Denisova, T.G.; Denisov, E.T. Dissociation energies of O-H bonds in natural antioxidants. Russ. Chem. Bull. 2008, 57, 1858–1866. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, X.-M.; Fry, A.J. Bond dissociation energies of antioxidants. Polym. Degrad. Stab. 1997, 57, 43–50. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, S. Comparative assessment of total phenolic content and in vitro antioxidant activities of bark and leaf methanolic extracts of Manilkara hexandra (Roxb.) Dubard. J. King Saud. Univ. Sci. 2020, 32, 643–647. [Google Scholar] [CrossRef]
- Franco, R.R.; Alves, V.H.M.; Zabisky, L.F.R.; Justino, A.B.; Martins, M.M.; Saraiva, A.L.; Goulart, L.R.; Espindola, F.S. Antidiabetic potential of Bauhinia forficata Link leaves: A non-cytotoxic source of lipase and glycoside hydrolases inhibitors and molecules with antioxidant and antiglycation properties. Biomed. Pharmacother. 2020, 123, 109798. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Wang, G.-Y.; Lin, J.-H.; Yen, G.-C. Antioxidant and anti-inflammatory activities and bioactive compounds of the leaves of Trichodesma khasianum clarke. Ind. Crops Prod. 2020, 151, 112447. [Google Scholar] [CrossRef]
- Cendrowski, A.; Kraśniewska, K.; Przybył, J.L.; Zielińska, A.; Kalisz, S. Antibacterial and Antioxidant Activity of Extracts from Rose Fruits (Rosa rugosa). Molecules 2020, 25, 1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saddiqe, Z.; Naeem, I.; Hellio, C.; Patel, A.V.; Abbas, G. Phytochemical profile, antioxidant and antibacterial activity of four Hypericum species from the UK. S. Afr. J. Bot. 2020, 133, 45–53. [Google Scholar] [CrossRef]
- Todorovic, V.; Milenkovic, M.; Vidovic, B.; Todorovic, Z.; Sobajic, S. Correlation between Antimicrobial, Antioxidant Activity, and Polyphenols of Alkalized/Nonalkalized Cocoa Powders. J. Food Sci. 2017, 82, 1020–1027. [Google Scholar] [CrossRef]
- Demenciano, S.d.C.; Silva, M.C.B.L.E.; Alexandrino, C.A.F.; Kato Junior, W.H.; Figueiredo, P.d.O.; Garcez, W.S.; Campos, R.P.; Guimarães, R.d.C.A.; Sarmento, U.C.; Bogo, D. Antiproliferative Activity and Antioxidant Potential of Extracts of Garcinia gardneriana. Molecules 2020, 25, 3201. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. [5] Measurement of oxygen radical absorbance capacity in biological samples. In Methods in Enzymology; Academic Press: London, UK, 1999; Volume 299, pp. 50–62. [Google Scholar]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; Volume 2, pp. 33–50. ISBN 9780128147740. [Google Scholar]
- Wang, Y.; Yang, M.; Lee, S.-G.; Davis, C.G.; Kenny, A.; Koo, S.I.; Chun, O.K. Plasma total antioxidant capacity is associated with dietary intake and plasma level of antioxidants in postmenopausal women. J. Nutr. Biochem. 2012, 23, 1725–1731. [Google Scholar] [CrossRef]
- Medini, F.; Bourgou, S.; Lalancette, K.; Snoussi, M.; Mkadmini, K.; Coté, I.; Abdelly, C.; Legault, J.; Ksouri, R. Phytochemical analysis, antioxidant, anti-inflammatory, and anticancer activities of the halophyte Limonium densiflorum extracts on human cell lines and murine macrophages. South Afr. J. Bot. 2015, 99, 158–164. [Google Scholar] [CrossRef]
- Mokrani, A.; Cluzet, S.; Madani, K.; Pakina, E.; Gadzhikurbanov, A.; Mesnil, M.; Monvoisin, A.; Richard, T. HPLC-DAD-MS/MS profiling of phenolics from different varieties of peach leaves and evaluation of their antioxidant activity: A comparative study. Int. J. Mass Spectrom. 2019, 445, 116192. [Google Scholar] [CrossRef]
- Vidal-Gutiérrez, M.; Robles-Zepeda, R.E.; Vilegas, W.; Gonzalez-Aguilar, G.A.; Torres-Moreno, H.; López-Romero, J.C. Phenolic composition and antioxidant activity of Bursera microphylla A. Gray. Ind. Crops Prod. 2020, 152, 112412. [Google Scholar] [CrossRef]
- An, F.; Yang, G.; Tian, J.; Wang, S. Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice. Neural Regen. Res. 2012, 7, 2565–2575. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khole, S.; Panat, N.A.; Suryawanshi, P.; Chatterjee, S.; Devasagayam, T.P.A.; Ghaskadbi, S. Comprehensive Assessment of Antioxidant Activities of Apigenin Isomers: Vitexin and Isovitexin. Free Rad. Antiox. 2016, 6, 155–166. [Google Scholar] [CrossRef]
- Deepha, V.; Praveena, R.; Sivakumar, R.; Sadasivam, K. Experimental and theoretical investigations on the antioxidant activity of isoorientin from Crotalaria globosa. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 121, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Park, C.M. Luteolin and luteolin-7-O-glucoside strengthen antioxidative potential through the modulation of Nrf2/MAPK mediated HO-1 signaling cascade in RAW 264.7 cells. Food Chem. Toxicol. 2014, 65, 70–75. [Google Scholar] [CrossRef]
- Marković, Z.; Jeremić, S.; Dimitrić Marković, J.; Stanojević Pirković, M.; Amić, D. Influence of structural characteristics of substituents on the antioxidant activity of some anthraquinone derivatives. Comput. Theor. Chem. 2016, 1077, 25–31. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Chuang, D.-Y. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Zarren, G.; Shafiq, N.; Arshad, U.; Rafiq, N.; Parveen, S.; Ahmad, Z. Copper-catalyzed one-pot relay synthesis of anthraquinone based pyrimidine derivative as a probe for antioxidant and antidiabetic activity. J. Mol. Struct. 2021, 1227, 129668. [Google Scholar] [CrossRef]
- Kosalec, I.; Kremer, D.; Locatelli, M.; Epifano, F.; Genovese, S.; Carlucci, G.; Randić, M.; Zovko Končić, M. Anthraquinone profile, antioxidant and antimicrobial activity of bark extracts of Rhamnus alaternus, R. fallax, R. intermedia and R. pumila. Food Chem. 2013, 136, 335–341. [Google Scholar] [CrossRef]
- Opretzka, L.C.F.; Espírito-Santo, R.F.d.; Nascimento, O.A.; Abreu, L.S.; Alves, I.M.; Döring, E.; Soares, M.B.P.; Velozo, E.d.S.; Laufer, S.A.; Villarreal, C.F. Natural chromones as potential anti-inflammatory agents: Pharmacological properties and related mechanisms. Int. Immunopharmacol. 2019, 72, 31–39. [Google Scholar] [CrossRef]
- Gecibesler, I.H.; Disli, F.; Bayindir, S.; Toprak, M.; Tufekci, A.R.; Sahin Yaglıoglu, A.; Altun, M.; Kocak, A.; Demirtas, I.; Adem, S. The isolation of secondary metabolites from Rheum ribes L. and the synthesis of new semi-synthetic anthraquinones: Isolation, synthesis and biological activity. Food Chem. 2021, 342, 128378. [Google Scholar] [CrossRef]
- Bindhu, J.H.; Satyanarayana, B.; Ramanjaneyulu, K.; Reddy, K.H.; Reddy, B.H. Synthesis, Docking and Biological activities of novel Chromone linked [1,2,3]-triazole derivatives. Chem. Data Collect. 2021, 32, 100651. [Google Scholar] [CrossRef]
- Barba, B.; Diaz, J.G.; Goedken, V.L.; Herz, W.; Dominguez, X.A. Unusual cassanes from a Chamaecrista species. Tetrahedron 1992, 48, 4725–4732. [Google Scholar] [CrossRef]
- Crockett, S.L.; Kunert, O.; Pferschy-Wenzig, E.-M.; Jacob, M.; Schuehly, W.; Bauer, R. Phloroglucinol and Terpenoid Derivatives from Hypericum cistifolium and H. galioides (Hypericaceae). Front. Plant Sci. 2016, 7, 961. [Google Scholar] [CrossRef] [Green Version]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 9781119964575. [Google Scholar]
- Chosson, E.; Chaboud, A.; Chulia, A.J.; Raynaud, J. A phloracetophenone glucoside from Rhododendron ferrugineum. Phytochemistry 1998, 47, 87–88. [Google Scholar] [CrossRef]
- Engels, C.; Schieber, A.; Gänzle, M.G. Sinapic acid derivatives in defatted Oriental mustard (Brassica juncea L.) seed meal extracts using UHPLC-DAD-ESI-MS n and identification of compounds with antibacterial activity. Eur. Food Res. Technol. 2012, 234, 535–542. [Google Scholar] [CrossRef]
- Oliveira, D.N.; Ferreira, M.S.; Catharino, R.R. Rapid and simultaneous in situ assessment of aflatoxins and stilbenes using silica plate imprinting mass spectrometry imaging. PLoS ONE 2014, 9, e90901. [Google Scholar] [CrossRef]
- Justesen, U. Collision-induced fragmentation of deprotonated methoxylated flavonoids, obtained by electrospray ionization mass spectrometry. J. Mass Spectrom. 2001, 36, 169–178. [Google Scholar] [CrossRef]
- Xia, B.; Li, J.; Mei, W.; Ding, L.; Xu, H.; Zhou, Y. Tandem mass spectrometry fragmentation of the protonated 2-(2-phenylethyl)chromones from agarwood: Radical ions versus non-radical ions. J. Mass Spectrom. 2013, 48, 979–982. [Google Scholar] [CrossRef]
- Su, S.; Wang, Y.; Bai, L.; Xia, B.; Li, X.; Tang, Y.; Xu, P.; Xue, M. Structural elucidation of in vivo metabolites of isobavachalcone in rat by LC-ESI-MS(n) and LC-NMR. J. Pharm. Biomed. Anal. 2015, 104, 38–46. [Google Scholar] [CrossRef]
- Li, H.-J.; Deinzer, M.L. Tandem Mass Spectrometry for Sequencing Proanthocyanidins. Anal. Chem. 2007, 79, 1739–1748. [Google Scholar] [CrossRef]
- Friedrich, W.; Eberhardt, A.; Galensa, R. Investigation of proanthocyanidins by HPLC with electrospray ionization mass spectrometry. Eur. Food Res. Technol. 2000, 211, 56–64. [Google Scholar] [CrossRef]
- Jaiswal, R.; Jayasinghe, L.; Kuhnert, N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC-MS. J. Mass Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef]
- Reis, A.; Domingues, P.; Ferrer-Correia, A.J.V.; Domingues, M.R.M. Tandem mass spectrometry of intact oxidation products of diacylphosphatidylcholines: Evidence for the occurrence of the oxidation of the phosphocholine head and differentiation of isomers. J. Mass Spectrom. 2004, 39, 1513–1522. [Google Scholar] [CrossRef]
- Ponomarev, D.; Takhistov, V. Thermochemistry of Organic and Heteroorganic Species. Part 19. Structural Aspects and Thermochemical Approach to Isomerization and Fragmentation of Negative Ions: Skeletal Rearrangements, Molecular Ion and Artifacts. ChemInform 2006, 37, 198–213. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Yang, Y.; Sjövall, J.; Lindgren, J.A. Electrospray-collision-induced dissociation mass spectrometry of mono-, di- and tri-hydroxylated lipoxygenase products, including leukotrienes of the B-series and lipoxins. Rapid Commun. Mass Spectrom. 1996, 10, 183–196. [Google Scholar] [CrossRef]
- Crevelin, E.J.; Crotti, A.E.M.; Zucchi, T.D.; Melo, I.S.; Moraes, L.A.B. Dereplication ofStreptomycessp. AMC 23 polyether ionophore antibiotics by accurate-mass electrospray tandem mass spectrometry. J. Mass Spectrom. 2014, 49, 1117–1126. [Google Scholar] [CrossRef]
- Fredenhagen, A.; Derrien, C.; Gassmann, E. An MS/MS library on an ion-trap instrument for efficient dereplication of natural products. Different fragmentation patterns for [M + H]+ and [M + Na]+ ions. J. Nat. Prod. 2005, 68, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.P.; Gates, P.J.; Wilkins, J.P.G.; Staunton, J. Fragmentation studies on lasalocid acid by accurate mass electrospray mass spectrometry. Analyst 2002, 127, 1224–1227. [Google Scholar] [CrossRef] [PubMed]
- Crotti, A.E.M.; Lopes, J.L.C.; Lopes, N.P. Triple quadrupole tandem mass spectrometry of sesquiterpene lactones: A study of goyazensolide and its congeners. J. Mass Spectrom. 2005, 40, 1030–1034. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Zhao, Z. Fragmentation study of simvastatin and lovastatin using electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2001, 36, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Gates, P.J.; Staunton, J.; Stinear, T.; Cole, S.T.; Leadlay, P.F.; Spencer, J.B. Identification using LC-MSn of co-metabolites in the biosynthesis of the polyketide toxin mycolactone by a clinical isolate of Mycobacterium ulcerans. Chem. Commun. 2003, 2822–2823. [Google Scholar] [CrossRef]
- Kleigrewe, K.; Aydin, F.; Hogrefe, K.; Piecuch, P.; Bergander, K.; Würthwein, E.-U.; Humpf, H.-U. Structure elucidation of new fusarins revealing insights in the rearrangement mechanisms of the Fusarium mycotoxin fusarin C. J. Agric. Food Chem. 2012, 60, 5497–5505. [Google Scholar] [CrossRef] [PubMed]
- Irwin, H.S.; Barneby, R.C. The American Cassiinae: A Synoptical Revision of Leguminosae Tribe Cassieae Subtribe Cassiinae in the New World; Scientific Publications Department: New York, NY, USA, 1982. [Google Scholar]
- Troalen, L.G.; Phillips, A.S.; Peggie, D.A.; Barran, P.E.; Hulme, A.N. Historical textile dyeing with Genista tinctoria L.: A comprehensive study by UPLC-MS/MS analysis. Anal. Methods 2014, 6, 8915–8923. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J. Negative ion electrospray high-resolution tandem mass spectrometry of polyphenols. J. Mass Spectrom. 2016, 51, 33–43. [Google Scholar] [CrossRef]
- Desta, K.T.; Kim, G.S.; Abd El-Aty, A.M.; Raha, S.; Kim, M.-B.; Jeong, J.H.; Warda, M.; Hacımüftüoğlu, A.; Shin, H.-C.; Shim, J.-H.; et al. Flavone polyphenols dominate in Thymus schimperi Ronniger: LC–ESI–MS/MS characterization and study of anti-proliferative effects of plant extract on AGS and HepG2 cancer cells. J. Chromatogr. B 2017, 1053, 1–8. [Google Scholar] [CrossRef]
- Ma, Y.L.; Li, Q.M.; Van den Heuvel, H.; Claeys, M. Characterization of flavone and flavonol aglycones by collision-induced dissociation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 1997, 11, 1357–1364. [Google Scholar] [CrossRef]
- Frański, R.; Matławska, I.; Bylka, W.; Sikorska, M.; Fiedorow, P.; Stobiecki, M. Differentiation of interglycosidic linkages in permethylated flavonoid glycosides from linked-scan mass spectra (B/E). J. Agric. Food Chem. 2002, 50, 976–982. [Google Scholar] [CrossRef]
- March, R.E.; Miao, X.-S. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom. 2004, 231, 157–167. [Google Scholar] [CrossRef]
- Hughes, R.J.; Croley, T.R.; Metcalfe, C.D.; March, R.E. A tandem mass spectrometric study of selected characteristic flavonoids. Int. J. Mass Spectrom. 2001, 210–211, 371–385. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Wu, W.; Yan, C.; Li, L.; Liu, Z.; Liu, S. Studies on the flavones using liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2004, 1047, 213–220. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of Flavonoids in Rhamnus davurica and Its Antiproliferative Activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, Y.; Zhang, Z.; Yang, C.; Tian, Y. Quasi-MSn identification of flavanone 7-glycoside isomers in Da Chengqi Tang by high performance liquid chromatography-tandem mass spectrometry. Chin. Med. 2009, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Su, W.; Zheng, Y.; Liu, H.; Li, P.; Zhang, W.; Liang, Y.; Bai, Y.; Peng, W.; Yao, H. UFLC-Q-TOF-MS/MS-Based Screening and Identification of Flavonoids and Derived Metabolites in Human Urine after Oral Administration of Exocarpium Citri Grandis Extract. Molecules 2018, 23, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Fu, J.; Yin, X.; Yang, C.; Zhang, X.; Wang, W.; Du, X.; Wang, Q.; Ni, J. Cassiae semen: A review of its phytochemistry and pharmacology (Review). Mol. Med. Rep. 2017, 16, 2331–2346. [Google Scholar] [CrossRef] [Green Version]
- El-Halawany, A.M.; Chung, M.H.; Nakamura, N.; Ma, C.-M.; Nishihara, T.; Hattori, M. Estrogenic and anti-estrogenic activities of Cassia tora phenolic constituents. Chem. Pharm. Bull. 2007, 55, 1476–1482. [Google Scholar] [CrossRef] [Green Version]
- Hatano, T.; Uebayashi, H.; Ito, H.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Phenolic constituents of Cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant Staphylococcus aureus. Chem. Pharm. Bull. 1999, 47, 1121–1127. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Fu, J.; Guo, H.; Tian, Y.; Xu, F.; Song, R.; Zhang, Z. Discrimination of crude and processed rhubarb products using a chemometric approach based on ultra fast liquid chromatography with ion trap/time-of-flight mass spectrometry. J. Sep. Sci. 2015, 38, 395–401. [Google Scholar] [CrossRef]
- Saito, N.; Tatsuzawa, F.; Nishiyama, A.; Yokoi, M.; Shigihara, A.; Honda, T. Acylated cyanidin 3-sambubioside-5-glucosides in Matthiola incana. Phytochemistry 1995, 38, 1027–1032. [Google Scholar] [CrossRef]
- Ye, M.; Han, J.; Chen, H.; Zheng, J.; Guo, D. Analysis of phenolic compounds in rhubarbs using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Wertz, J.; Degano, I.; Aceto, M.; Khan, M.I.; Quye, A. Analytical methods for determination of anthraquinone dyes in historical textiles: A review. Anal. Chim. Acta 2019, 1083, 58–87. [Google Scholar] [CrossRef]


| Samples | AA (mmol TE/g) | TP (mg GAE/g) | TF (mg CE/g) |
|---|---|---|---|
| ORAC Value | |||
| Ethanolic extract (EE) | 4.29 ± 0.20 | 131.08 ± 0.95 | 9.31 ± 0.26 |
| Fr-MeOH | 6.59 ± 0.27 | 157.38 ± 13.20 | 9.58 ± 0.12 |
| Fr-OAcEt | 9.44 ± 0.09 | 302.21 ± 3.10 | 21.9 ± 0.10 |
| Peak | RT (min) | MF | [M–H]– | Product Ions MS/MS | Putative Identity | Reference Spectrum ID | EE | Fr-MeOH | Fr-OAcEt | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Theoretical Accurate m/z | Experimental Accurate m/z | Error ppm | |||||||||
| 1 | 4.10 | C10H10O5 | 209.0450 | 209.0446 | 1.9 | 191, 165, 123 | 2,4-diacetylphloroglucinol | b CCMSLIB00004693587 | x | - | x |
| 2 | 4.36 | C21H20O11 | 447.0927 | 447.0925 | 0.4 | 429, 369, 357, 327, 299, 285 | orientin | c FIO00705 | x | x | - |
| 3 | 4.48 | C21H20O11 | 447.0927 | 447.0930 | 0.7 | 429, 369, 357, 327, 299, 285 | isoorientin | c FIO00715 | x | x | - |
| 4 | 4.61 | C22H22O13 | a 493.0982 | a 493.0985 | 0.6 | 447 [M – H]–, 369, 357, 327, 299, 285 | 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-8-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]chromen-4-one | bCCMSLIB00000846053 | x | x | - |
| 5 | 4.80 | C11H12O5 | 223.0606 | 223.0600 | 2.7 | 208, 193, 179, 164, 149 | sinapic acid | bCCMSLIB00005738417 | x | - | x |
| 6 | 5.02 | C21H20O10 | 431.0978 | 431.0980 | 0.5 | 413, 353, 341, 311, 283, 269 | vitexin | dFIO00915 | x | x | - |
| 7 | 5.12 | C21H20O10 | 431.0978 | 431.0980 | 0.5 | 413, 353, 341, 311, 283, 269 | isovitexin | dFIO00915 | x | x | - |
| 8 | 5.40 | C21H20O11 | 447.0927 | 447.0925 | 0.4 | 357, 339, 327, 311, 299, 285, 255, 151, 133 | luteolin-7-O-glucoside | cVFNPL-QEHF013327 | x | x | - |
| 9 | 5.68 | C13H14O4 | 233.0814 | 233.0810 | 1.7 | 215, 189, 174 161, 149 | Aloesol | [49] | x | x | x |
| 10 | 5.93 | C15H12O6 | 287.0556 | 287.0551 | 1.7 | 269, 259, 243, 201, 151 | dihydrokaempferol | bCCMSLIB00004684095 | x | - | x |
| 11 | 6.27 | C9H16O4 | 187.0970 | 187.0965 | 2.7 | 181, 125 | azelaic acid | c KO000123 | x | - | x |
| 12 | 6.36 | C11H10O3 | 189.0552 | 189.0545 | 3.7 | 174, 161, 149 | 7-hydroxy-2,5-dimethyl-4H-chromen-4-one | [51] | x | x | x |
| 13 | 6.40 | C10H8O4 | 191.0344 | 191.0360 | 8.4 | 176, 149 | 1H-2-benzopyran-1-one, 6,8-dihydroxy-3-methyl | c VF-NPL-QEHF001189 | x | - | x |
| 14 | 6.61 | C22H22O13 | 461.1084 | 461.1072 | 2.6 | 446, 299 | 5-[4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]chromen-4-one | b CCMSLIB00004717546 | x | x | - |
| 15 | 7.16 | C26H34O14 | 569.1870 | 569.1868 | 0.4 | 245, 230, 215 | torachrysone-8-hexosyl-hexoside | e HOV19-D | x | x | - |
| 16 | 7.57 | C25H32O13 | 539.1765 | 539.1760 | 0.9 | 245, 230, 215 | torachrysone-8-hexosyl-pentoside isomer | e HOV18-C | x | x | - |
| 17 | 7.72 | C15H10O6 | 285.0399 | 285.0396 | 1.1 | 241, 217, 133 | luteolin | b CCMSLIB00004718183 | x | x | x |
| 18 | 7.80 | C25H32O13 | 539.1765 | 539.1770 | 0.9 | 245, 230, 215 | torachrysone-8-hexosyl-pentoside isomer | e HOV18-C | x | x | - |
| 19 | 8.10 | C14H12O3 | 227.0708 | 227.0709 | 0.4 | 185, 157 | resveratrol | d BML00673 | x | - | x |
| 20 | 8.12 | C14H14O5 | 261.0763 | 261.0762 | 0.4 | 246, 231, 217, 203 | rutaretin isomer | [49] | x | - | x |
| 21 | 8.46 | C30H26O13 | 593.1295 | 593.1294 | 0.2 | 575, 447, 429, 369, 357, 327 | orientin-O-hexoside | [43] | x | x | - |
| 22 | 8.74 | C15H12O5 | 271.0606 | 271.0601 | 1.8 | 253, 227, 199, 177, 151 | naringenin | b CCMSLIB00004719909 | x | - | x |
| 23 | 8.86 | C15H10O5 | 269.0450 | 269.0447 | 1.1 | 225, 151 | apigenin | b CCMSLIB00005787970 | x | - | x |
| 24 | 9.22 | C16H12O6 | 299.0556 | 299.0554 | 0.7 | 284, 256, 227, 199 | carviolin isomer | b CCMSLIB00004697562 | x | x | x |
| 25 | 9.25 | C30H26O12 | 577.1346 | 577.1355 | 1.6 | 431, 413, 353, 341, 311, 283, 269 | isovitexin-O-pentoside | [43] | x | x | - |
| 26 | 9.90 | C14H14O5 | 261.0763 | 261.0758 | 1.9 | 246, 231, 217, 203 | rutaretin isomer | [49] | x | - | x |
| 27 | 9.97 | C18H32O5 | 327.2171 | 327.2165 | 1.8 | 291, 229, 211, 183, 171 | 9,12,13-trihydroxy-10(E),15(Z)- octadecadienoic acid | [52] | x | x | x |
| 28 | 10.2 | C12H20O4 | 227.1283 | 227.1281 | 0.9 | 209, 183, 165 | butanedioic acid, 2-(4,4-dimethyl-2-methylenepentyl) isomer | b CCMSLIB00003138678 | x | - | x |
| 29 | 10.3 | C14H14O5 | 261.0763 | 261.0754 | 3.4 | 246, 231, 217, 203 | rutaretin isomer | [49] | x | - | x |
| 30 | 10.4 | C12H20O4 | 227.1283 | 227.1281 | 0.9 | 209, 183, 165 | butanedioic acid, 2-(4,4-dimethyl-2-methylenepentyl) isomer | b CCMSLIB00003138678 | x | - | x |
| 31 | 10.8 | C18H34O5 | 329.2328 | 329.2320 | 2.4 | 311, 293, 229, 211, 183, 171 | 9,12,13-trihydroxyoctadec-10-enoic acid | e JTY13-R[52] | x | x | - |
| 32 | 10.9 | C14H10O5 | 257.0450 | 257.0442 | 3.1 | 213, 171, 159, 137 | norlichexanthone | c VFNPL-QEHF017949 | x | - | x |
| 33 | 11.2 | C16H12O6 | 299.0556 | 299.0550 | 2.0 | 284, 256, 227, 199 | carviolin isomer | b CCMSLIB00004697562 | x | x | - |
| 34 | 14.3 | C15H10O5 | 269.0450 | 269.0444 | 2.2 | 241, 225 | emodin | b CCMSLIB00004702275 | x | - | x |
| 35 | 16.6 | C18H30O3 | 293.2117 | 293.2115 | 0.7 | 275, 256, 224, 195 | hydroxyoctadecatrienoic acid | d IA000196 13-HOTrE | x | - | x |
| Radical Species | BDE/kcal mol−1 |
|---|---|
| Chromones | |
| aloesol (9) | |
| 7-O• | 89.3 |
| 90.3 a | |
| 90.0 b | |
| 12-O• | 104.3 |
| 105.1 a | |
| 105.1 b | |
| 7-hydroxy-2,5-dimethyl-4H-chromen-4-one (12) | |
| 7-O• | 83.5 |
| 89.3 a | |
| 120.8 b | |
| Flavone | |
| luteolin (17) | |
| 5-O• | 103.8 |
| 6-O• | 90.5 |
| 4′-O• | 85.9 |
| 5′-O• | 78.0 |
| Coumarin and anthraquinone isomers | |
| rutaretin (20, 26, 29) | |
| (CH3)-O• | 107.3 |
| 8-O• | 121.5 |
| carviolin (24, 33) | |
| 1-O• | 108.1 |
| 3-O• | 90.7 |
| 6-CH2-O• | 100.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, P.; Quirós-Guerrero, L.; Muribeca, A.; Reis, J.; Pamplona, S.; Lima, A.H.; Trindade, M.; Silva, C.; Souza, J.N.S.; Boutin, J.A.; et al. Constituents of Chamaecrista diphylla (L.) Greene Leaves with Potent Antioxidant Capacity: A Feature-Based Molecular Network Dereplication Approach. Pharmaceutics 2021, 13, 681. https://doi.org/10.3390/pharmaceutics13050681
Gomes P, Quirós-Guerrero L, Muribeca A, Reis J, Pamplona S, Lima AH, Trindade M, Silva C, Souza JNS, Boutin JA, et al. Constituents of Chamaecrista diphylla (L.) Greene Leaves with Potent Antioxidant Capacity: A Feature-Based Molecular Network Dereplication Approach. Pharmaceutics. 2021; 13(5):681. https://doi.org/10.3390/pharmaceutics13050681
Chicago/Turabian StyleGomes, Paulo, Luis Quirós-Guerrero, Abraão Muribeca, José Reis, Sônia Pamplona, Anderson H. Lima, Mariele Trindade, Consuelo Silva, Jesus N. S. Souza, Jean A. Boutin, and et al. 2021. "Constituents of Chamaecrista diphylla (L.) Greene Leaves with Potent Antioxidant Capacity: A Feature-Based Molecular Network Dereplication Approach" Pharmaceutics 13, no. 5: 681. https://doi.org/10.3390/pharmaceutics13050681
APA StyleGomes, P., Quirós-Guerrero, L., Muribeca, A., Reis, J., Pamplona, S., Lima, A. H., Trindade, M., Silva, C., Souza, J. N. S., Boutin, J. A., Wolfender, J. -L., & Silva, M. (2021). Constituents of Chamaecrista diphylla (L.) Greene Leaves with Potent Antioxidant Capacity: A Feature-Based Molecular Network Dereplication Approach. Pharmaceutics, 13(5), 681. https://doi.org/10.3390/pharmaceutics13050681