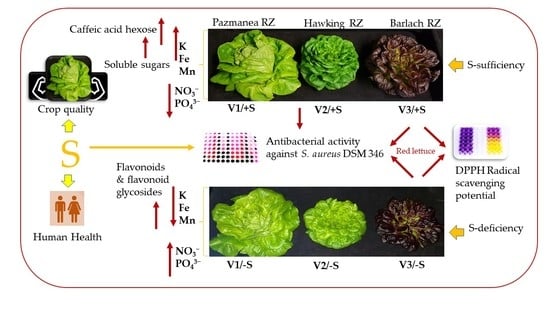
Comparative Metabolite Profile, Biological Activity and Overall Quality of Three Lettuce (Lactuca sativa L., Asteraceae) Cultivars in Response to Sulfur Nutrition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Determination of Inorganic Anions, Organic Acids and Water-Soluble Sugars
2.3. Mineral Analyses Using ICP-MS
2.4. Determination of Sulfur and Nitrogen Concentrations Using an Elemental Analyzer
2.5. LC-DAD-MS Analyses
2.6. Antibacterial Assay
2.7. DPPH Radical Scavenging Activity
2.8. Statistical Analyses
3. Results and Discussion
3.1. Plant Biomass
3.2. Free Inorganic Anions, WSS, and OAs
3.3. Elemental Composition
3.4. Comparative Secondary Metabolite Profile of the Three Lettuce Cultivars under S-Sufficient (+S) and S-Deficient (−S) Conditions
3.5. Antibacterial Activity
3.6. Free radical Scavanging (Antioxidant) Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdalla, M.A.; Sulieman, S.; Mühling, K.H. Regulation of selenium/sulfur interactions to enhance chemopreventive effects: Lessons to learn from Brassicaceae. Molecules 2020, 25, 5846. [Google Scholar] [CrossRef] [PubMed]
- Meschede, C.A.C.; Abdalla, M.A.; Mühling, K.H. Sulfur but not nitrogen supply increases the ITC/Nitrile ratio in Pak Choi (Brassica rapa subsp. Chinensis (L.) Hanelt). J. Appl. Bot. Food Qual. 2020, 93, 95–104. [Google Scholar]
- Kopriva, S.; Malagoli, M.; Takahashi, H. Sulfur nutrition: Impacts on plant development, metabolism, and stress responses. J. Exp. Bot. 2019, 70, 4069–4073. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; Mühling, K.H. Plant-derived sulfur containing natural products produced as a response to biotic and abiotic stresses: A review of their structural diversity and medicinal importance. J. Appl. Bot. Food Qual. 2019, 92, 204–215. [Google Scholar]
- Nimni, M.E.; Han, B.; Cordoba, F. Are we getting enough sulfur in our diet? Nutr. Metab. 2007, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Van de Poll, M.C.; Dejong, C.H.C.; Soeters, P.B. Adequate range for sulfur-containing amino acids and biomarkers for their excess: Lessons from enteral and parenteral nutrition. J. Nutr. 2006, 136, 1694s–1700s. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Liu, N.; Zhang, P.; Guo, Y.; Zhang, Y.; Zhao, Z.; Luan, Y.; Li, S.; Cai, J.; Cao, J. Synthetic methods of disulfide bonds applied in drug delivery systems. Curr. Org. Chem. 2016, 20, 1477–1489. [Google Scholar] [CrossRef]
- Mou, B. Lettuce. In Handbook of Plant Breeding, Vegetables I: Asteraceae, -Brassicaceae, Chenopodicaceae, and Cucurbitaceae; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; Volume I, pp. 75–116. [Google Scholar]
- FAOSTAT—Food & Agriculture Organization, Statistics Division. Lettuce (with Chicory) Production in 2015; Countries/Regions/Production Quantity from Pick Lists. 2015. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 13 November 2019).
- Transparency Market Research 2017. Available online: https://www.prnewswire.com/news-releases/hydroponic-vegetables-market-to-reach-us121065-mn-by-2025growing-concerns-about-food-security-ups-demand-for-hydroponics-says-tmr-626494961.html (accessed on 11 September 2020).
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compost. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Dupont, M.S.; Mondin, Z.; Williamson, G.; Price, K.R. Effect of variety, processing, and storage on the flavonoid glycoside content and composition of lettuce and endive. J. Agric. Food Chem. 2000, 48, 3957–3964. [Google Scholar] [CrossRef]
- Liu, X.; Ardo, S.; Bunning, M.; Parry, J.; Zhou, K.; Stushnoff, C.; Kendall, P. Total phenolic content and DPPH radical scavenging activity of lettuce (Lactuca sativa L.) grown in Colorado. LWT-Food Sci. Technol. 2007, 40, 552–557. [Google Scholar] [CrossRef]
- Padilla-Gonzalez, G.F.; dos Santos, F.A.; Da Costa, F.B. Sesquiterpene lactones: More than protective plant compounds with high toxicity. Crit. Rev. Plant Sci. 2016, 35, 18–37. [Google Scholar] [CrossRef]
- Sessa, R.A.; Bennett, M.H.; Lewis, M.J.; Mansfield, J.W.; Beale, M.H. Metabolite profiling of sesquiterpene lactones from lactuca species: Major latex components are novel oxalate and sulfate conjugates of lactucin and its derivatives. J. Biol. Chem. 2000, 275, 26877–26884. [Google Scholar] [CrossRef]
- Mai, F.; Glomb, M.A. Isolation of phenolic compounds from iceberg lettuce and impact on enzymatic browning. J. Agric. Food Chem. 2013, 61, 2868–2874. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Meschede, C.A.C.; Mühling, K.H. Selenium foliar application alters patterns of glucosinolatehydrolysis products of pak choi Brassica rapa L. var. Chinensis. Sci. Hortic. 2020, 273, 109614. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef]
- Cometti, N.N.; Bremenkamp, D.M.; Galon, K.; Hell, L.R.; Zanotelli, M.F. Cooling and concentration of nutrient solution in hydroponic lettuce crop. Hortic. Bras. 2013, 31, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Cataldi, T.R.; Margiotta, G.; Iasi, L.; Di Chio, B.; Xiloyannis, C.; Bufo, S.A. Determination of sugar compounds in olive plant extracts by anion-exchange chromatography with pulsed amperometric detection. Anal. Chem. 2000, 72, 3902–3907. [Google Scholar] [CrossRef]
- Jezek, M.; Geilfus, C.M.; Bayer, A.; Mühling, K.H. Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 2015, 5, 781. [Google Scholar] [CrossRef] [Green Version]
- Zörb, C.; Mühling, K.H.; Hasler, M.; Gödde, V.; Niehaus, K.; Becker, D.; Geilfus, C.M. Metabolomic responses in grain, ear, and straw of winter wheat under increasing sulfur treatment. J. Plant. Nutr. Soil Sci. 2013, 176, 964–970. [Google Scholar] [CrossRef]
- Kopriva, S.; Talukdar, D.; Takahashi, H.; Hell, R.; Sirko, A.; D’Souza, S.F.; Talukdar, T. Editorial: Frontiers of sulfur metabolism in plant growth, development, and stress response. Front. Plant Sci. 2016, 6, 1220. [Google Scholar] [CrossRef] [Green Version]
- Hawkesford, M.J. Plant responses to sulphur deficiency and the genetic manipulation of sulphate transporters to improve S-utilization efficiency. J. Exp. Bot. 2000, 51, 131–138. [Google Scholar] [CrossRef]
- Resurreccion, A.P.; Makino, A.; Bennett, J.; Mae, T. Effects of sulfur nutrition on the growth and photosynthesis of rice. Soil Sci. Plant Nutr. 2012, 47, 611–620. [Google Scholar] [CrossRef]
- Etienne, P.; Sorin, E.; Maillard, A.; Gallardo, K.; Arkoun, M.; Guerrand, J.; Cruz, F.; Yvin, J.-C.; Ourry, A. Assessment of sulfur deficiency under field conditions by single measurements of sulfur, chloride and phosphorus in mature leaves. Plants 2018, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Cuppettz, S.L.; McCluskey, M.M.; Paparozzi, E.T.; Parkhursp, A. Nitrogen and sulfur effects on leaf lettuce quality. J. Food Qual. 1999, 22, 33–373. [Google Scholar]
- Senizza, B.; Zhang, L.; Miras-Moreno, B.; Righetti, L.; Zengin, G.; Ak, G.; Bruni, R.; Lucini, L.; Sifola, M.I.; El-Nakhel, C.; et al. The strength of the nutrient solution modulates the functional profile of hydroponically grown lettuce in a genotype-dependent manner. Foods 2020, 9, 1156. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995; p. 889. [Google Scholar]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition; Springer: Dordrecht, The Netherlands, 2001; p. 673. [Google Scholar]
- Veliz, C.G.; Roberts, I.N.; Criado, M.V.; Caputo, C. Sulphur deficiency inhibits nitrogen assimilation and recycling in barley plants. Biol. Plant. 2017, 61, 675–684. [Google Scholar] [CrossRef]
- Anjana, S.U.; Iqbal, M.; Abrol, Y.P. Are nitrate concentrations in leafy vegetables within safe limits? Curr. Sci. 2007, 92, 355–360. [Google Scholar]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.P.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Calvo, M.S.; Moshfegh, A.J.; Tucker, K.L. Assessing the health impact of phosphorus in the food supply: Issues and considerations. Adv. Nutr. 2014, 5, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Chadwick, M.; Gawthrop, F.; Michelmore, R.W.; Wagstaff, C.; Methven, L. Perception of bitterness, sweetness and liking of different genotypes of lettuce. Food Chem. 2016, 197, 66–74. [Google Scholar] [CrossRef]
- Rendig, V.V.; Oputa, C.; McComb, E.A. Effects of sulfur deficiency on non-protein nitrogen, soluble sugars, and N/S ratios in young corn (Zea mays L.) plants. Plant Soil 1976, 44, 423–437. [Google Scholar] [CrossRef]
- Nikiforova, V.J.; Kopka, J.; Tolstikov, V.; Fiehn, O.; Hopkins, L.; Hawkesford, M.L.; Hesse, H.; Hoefgen, R. Systems re-balancing of metabolism in response to sulfur deprivation, as revealed by metabolome analysis of Arabidopsis plants. Plant Physiol. 2005, 138, 304–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J.G.; Walker, R.P. The organic acids that are accumulated in the flesh of fruits: Occurrence, metabolism and factors affecting their contents—A review. Rev. Chapingo Ser. Hortic. 2015, 21, 97–128. [Google Scholar] [CrossRef]
- Reich, M.; Shahbaz, M.; Prajapati, D.H.; Parmar, S.; Hawkesford, M.J.; De Kok, L.J. Interactions of sulfate with other nutrients As revealed by H2S fumigation of Chinese cabbage. Front Plant Sci. 2016, 7, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruulsema, T.W.; Heffer, P.; Welch, R.M.; Cakmak, I.; Moran, K. Fertilizing Crops to Improve Human Health: A Scientific Review, 1st ed.; IPNI: Norcross, GA, USA; IFA: Paris, France, 2012. [Google Scholar]
- Yu, Z.; Juhasz, A.; Islam, S.; Diepeveen, D.; Zhang, J.; Wang, P.; Ma, W. Impact of mid-season sulphur deficiency on wheat nitrogen metabolism and biosynthesis of grain protein. Sci. Rep. 2018, 8, 2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westermarm, D.T. Indexes of sulfur deficiency in Alfalfa. II. Plant analyses. Agron. J. 1975, 67, 265–268. [Google Scholar] [CrossRef]
- Thomas, S.G.; Bilsborrow, P.E.; Hocking, T.J.; Bennett, J. Effect of sulphur deficiency on the growth and metabolism of sugar beet (Beta vulgaris cv. Druid). J. Sci. Food Agric. 2000, 80, 2057–2062. [Google Scholar] [CrossRef]
- Klikocka, H.; Marks, M. Sulphur and nitrogen fertilization as a potential means of agronomic biofortification to improve the content and uptake of microelements in spring wheat grain DM. J. Chem. 2018, 2018, 9326820. [Google Scholar] [CrossRef]
- Zuchi, S.; Watanabe, M.; Hubberten, H.M.; Bromke, M.; Osorio, S.; Fernie, A.R.; Celletti, S.; Paolacci, A.R.; Catarcione, G.; Ciaffi, M.; et al. The interplay between sulfur and iron nutrition in tomato. Plant Physiol. 2015, 169, 2624–2639. [Google Scholar] [CrossRef] [Green Version]
- Wen, W.; Li, K.; Alseekh, S.; Omranian, N.; Zhao, L.; Zhou, Y.; Xiao, Y.; Jin, M.; Yang, N.; Liu, H.; et al. Genetic determinants of the network of primary metabolism and their relationships to plant performance in a maize recombinant inbred line population. Plant Cell 2015, 27, 1839–1856. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Sun, X.; Zhang, Z.; Ni, Y.; Zhang, Q.; Liang, X.; Xiao, H.; Chen, J.; Tokuhisa, J.G. Metabolite profiling of Arabidopsis seedlings in response to exogenous sinalbin and sulfur deficiency. Phytochemistry 2011, 72, 1767–1778. [Google Scholar] [CrossRef]
- Nikiforova, V.; Freitag, J.; Kempa, S.; Adamik, M.; Hesse, H.; Hoefgen, R. Transcriptome analysis of sulfur depletion in Arabidopsis thaliana: Interlacing of biosynthetic pathways provides response specificity. Plant J. 2003, 33, 633–650. [Google Scholar] [CrossRef]
- Haak, D.C.; Fukao, T.; Grene, R.; Hua, Z.; Ivanov, R.; Perrella, G.; Li, S. Multilevel regulation of abiotic stress responses in plants. Front. Plant Sci. 2017, 8, 1564. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- GNPS Database. Available online: https://gnps.ucsd.edu/ProteoSAFe/static/gnps-splash.jsp (accessed on 21 October 2020).
- MoNA Database: MassBank of North America. Available online: https://mona.fiehnlab.ucdavis.edu/ (accessed on 21 October 2020).
- Abu-Reidah, I.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L.) by HPLC–DAD-ESI-QTOF-MS. Food Chem. 2013, 141, 2269–2277. [Google Scholar] [CrossRef]
- Assefa, A.D.; Choi, S.; Lee, J.-E.; Sung, J.-S.; Hur, O.-S.; Ro, N.-Y.; Lee, H.-S.; Jang, S.-W.; Rhee, J.-H. Identification and quantification of selected metabolites in differently pigmented leaves of lettuce (Lactuca sativa L.) cultivars harvested at mature and bolting stages. BMC Chem. 2019, 13, 56. [Google Scholar] [CrossRef] [Green Version]
- ReSpect Database: RIKEN MSn Spectral Database for Phytochemicals. Available online: http://spectra.psc.riken.jp/ (accessed on 21 October 2020).
- Materska, M.; Olszówka, K.; Chilczuk, B.; Stochmal, A.; Pecio, L.; Pacholczyk-Sienicka, B.; Piacente, S.; Pizza, C.; Masullo, M. Polyphenolic profiles in lettuce (Lactuca sativa L.) after CaCl2 treatment and cold storage. Eur. Food Res. Technol. 2019, 245, 733–744. [Google Scholar] [CrossRef] [Green Version]
- Bonta, R.K. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs). J. Pharm. Anal. 2017, 7, 349–364. [Google Scholar]
- Ju, W.-T.; Kwon, O.-C.; Kim, H.-B.; Sung, G.-B.; Kim, H.-W.; Kim, Y.-S. Qualitative and quantitative analysis of flavonoids from 12 species of Korean mulberry leaves. J. Food Sci. Technol. 2018, 55, 1789–1796. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Zhou, J.; Ren, T.; Du, H.; Liu, H.; Li, Y.; Zhang, C. Salt stress decreases seedling growth and development but increases quercetin and kaempferol content in Apocynum venetum. Plant Biol. 2020, 22, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, S.; Cheng, Y.; Peng, Z.; Han, J. Transcriptome profiling of anthocyanin-related genes reveals effects of light intensity on anthocyanin biosynthesis in red leaf lettuce. PeerJ 2018, 6, e4607. [Google Scholar] [CrossRef]
- Weld, J.T.; Gunther, A. The antibacterial properties of sulfur. J. Exp. Med. 1947, 85, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Libenson, L.; Hadley, F.P.; McIlroy, A.P.; Wetzel, V.M.; Mellon, R.R. Antibacterial effect of elemental sulfur. J. Infect. Dis. 1953, 93, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Wang, Z. Cysteine potentiates bactericidal antibiotics activity against Gram-negative bacterial persisters. Infect. Drug Resist. 2020, 13, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Jadoun, J.; Yazbak, A.; Rushrush, S.; Rudy, A.; Azaizeh, H. Identification of a new antibacterial sulfur compound from Raphanus sativus seeds. Evid. Based Complement. Altern. Med. 2016, 2016, 9271285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, E.; Ferreira, I.C.F.R.; Barros, L.; Carvalho, A.M.; Soares, G.; Henriques, M. Antibacterial potential of Northeastern Portugal wild plant extracts and respective phenolic compounds. BioMed Res. Int. 2014, 2014, 814590. [Google Scholar] [CrossRef] [Green Version]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, cafeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. BioMed Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996, 96, 1027–1039. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Beek, T.A.V. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, J.; Ma, Y.; Sun, X.; Li, D.; Li, L.; Bai, H.; Xin, G.; Meng, X. Composition and antioxidant activity of anthocyanins from Aronia melanocarpa cultivated in Haicheng, Liaoning, China. Food Biosci. 2019, 30, 100413. [Google Scholar] [CrossRef]
- Cheng, D.M.; Pogrebnyak, N.; Kuhn, P.; Krueger, C.G.; Johnson, W.D.; Raskin, I. Development and phytochemical characterization of high polyphenol red lettuce with anti-diabetic properties. PLoS ONE 2014, 9, e91571. [Google Scholar] [CrossRef] [Green Version]
- Bellocco, E.; Barreca, D.; Laganà, G.; Calderaro, A.; El Lekhlifi, Z.; Chebaibi, S.; Smeriglio, A.; Trombetta, D. Cyanidin-3-O-galactoside in ripe pistachio (Pistachia vera L. variety Bronte) hulls: Identification and evaluation of its antioxidant and cytoprotective activities. J. Funct. Foods 2016, 27, 376–385. [Google Scholar] [CrossRef]
- Gould, K.S. Nature’s swiss army knife: The diverse protective roles of anthocyanins in leaves. J. Biomed. Biotechnol. 2004, 5, 314–320. [Google Scholar] [CrossRef] [Green Version]







| Treatments | ||||||
|---|---|---|---|---|---|---|
| V1/+S | V1/−S | V2/+S | V2/−S | V3/+S | V3/−S | |
| Anions | ||||||
| Cl− | 0.47 ± 0.08 b | 13.4 ± 0.96 a | 0.49 ± 0.06 b | 16.2 ± 1.12 a | 0.57 ± 0.02 b | 14.9 ± 0.41 a |
| SO42− | 1.35 ± 0.07 a | 0.21 ± 0.03 b | 1.42 ± 0.17 a | 0.26 ± 0.03 b | 1.33 ± 0.18 a | 0.54 ± 0.13 b |
| NO3− | 0.84 ± 0.17 b | 1.35 ± 0.31 b | 2.36 ± 0.50 b | 5.67 ± 0.28 a | 1.65 ± 0.54 b | 1.78 ± 0.28 b |
| PO43− | 3.86 ± 0.15 b | 5.58 ± 0.62 ab | 4.41 ± 0.30 b | 6.75 ± 0.47 a | 5.30 ± 0.27 ab | 4.99 ± 0.28 ab |
| Soluble sugars | ||||||
| Glucose | 5.07 ± 0.22 b | 2.14 ± 0.27 c | 4.59 ± 0.39 b | 1.73 ± 0.15 c | 7.29 ± 0.17 a | 8.37 ± 0.62 a |
| Fructose | 5.85 ± 0.09 a | 5.66 ± 0.39 a | 3.66 ± 0.492 a | 3.58 ± 0.34 a | 4.15 ± 0.20 a | 4.03 ± 0.33 a |
| Sucrose | 4.39 ± 0.39 a | 2.50± 0.21 bc | 4.40 ± 0.31 ab | 2.41 ± 0.16 c | 4.52± 0.37 a | 3.84 ± 0.21 abc |
| Organic acids | ||||||
| Malic acid | 13.82 ± 0.49 a | 10.09 ± 0.5 b | 14.47 ± 0.58 a | 11.37 ± 0.18 b | 9.74 ± 0.37 b | 9.628 ± 0.516 b |
| Oxalic acid | 1.62 ± 0.76 a | 1.30 ± 0.04 ab | 1.67 ± 0.13 a | 1.21 ± 0.07 ab | 1.41 ± 0.04 ab | 1.16 ± 0.10 b |
| Citric acid | 1.47 ± 0.13 a | 1.30 ± 0.10 a | 1.39 ± 0.12 a | 1.35 ± 0.17 a | 1.09 ± 0.07 a | 1.19 ± 0.10 a |
| Treatments | ||||||
|---|---|---|---|---|---|---|
| V1/+S | V1/−S | V2/+S | V2/−S | V3/+S | V3/−S | |
| Macronutrients | ||||||
| P | 8.88 ± 0.22 bc | 9.01 ± 0.29 b | 13.0 ± 0.06 a | 12.5 ± 0.24 a | 7.76 ± 0.12 cd | 7.08 ± 0.18 d |
| Mg | 3.38 ± 0.21 b | 2.75 ± 0.21 bc | 4.44 ± 0.08 a | 4.40 ± 0.15 a | 2.60 ± 0.08 c | 2.65 ± 0.16 bc |
| K | 58.8 ± 0.95 ab | 44.0 ± 1.49 c | 65.1 ± 2.4 a | 53.4 ± 1.38 b | 43.3 ± 1.05 c | 40.13 ± 2.08 c |
| Ca | 5.09 ± 0.33 b | 5.04 ± 0.37 b | 9.52 ± 0.10 a | 9.78 ± 0.11 a | 5.72 ± 0.20 b | 5.77 ± 0.14 b |
| N | 45.94 ± 1.6 bc | 51.31 ± 0.90 a | 43.51 ± 0.77 cd | 49.38 ± 1.04 ab | 40.43 ± 0.68 d | 45.23 ± 0.32 bcd |
| S | 4.33 ± 0.05 a | 1.63 ± 0.02 e | 3.89 ± 0.02 b | 1.90 ± 0.02 d | 3.83 ± 0.01 b | 2.14 ± 0.008 c |
| N/S ratio | 10.60 ± 0.27 d | 31.40 ± 0.28 a | 11.07 ± 0.23 d | 25.16 ± 0.46 b | 10.56 ± 0.16 d | 21.01 ± 0.27 c |
| Micronutrients | ||||||
| Mn | 340.3 ± 1.3 c | 255.0 ± 1.2 d | 441.0 ± 1.4 a | 415.5 ± 0.9 b | 251.7 ± 0.5 d | 238.3 ± 0.4 e |
| Fe | 142.5 ± 1.9 a | 132.8 ± 1.6 b | 149.7 ± 1.1 a | 147.5 ± 1.3 a | 127.8 ± 1.9 b | 128.0 ± 1.7 b |
| Cu | 8.42 ± 0.16 c | 8.57 ± 0.17 c | 14. 89 ± 0.28 a | 14.84 ± 0.19 a | 12.0 ± 0.13 b | 12.14 ± 0.25 b |
| Zn | 78.49 ± 2.1 c | 84.43 ± 2.5 bc | 94.96 ± 1.0 b | 110.80 ± 3.7 a | 77.57 ± 1.3 c | 74.87 ± 1.9 c |
| Compound | MW | Class | Cultivar | m/z | tR (min) | Mode | Ref |
|---|---|---|---|---|---|---|---|
| Lactucin | 276 | Sesquiterpene lactone | V1 | 277.1 | 4.5 | Pos. | [52] |
| Dihydrolactucopicrin | 412 | Sesquiterpene lactone | All | 413.1 | 7.3 | Pos. | [52] |
| Lactucopicrin | 410 | Sesquiterpene lactone | V1, V2, V3 | 411.1 | 7.3, 7.4 | Pos. | [53] |
| Cyanidin 3-O-galactoside | 448 | Anthocyanin glycoside | V3 | 449.1 | 4.1 | Pos. | [52] |
| Luteolin-7-glucuronide | 462 | Flavonoid-7-O-glucuronide | All | 463.1 | 5.5 | Pos. | [54] |
| Dicaffeoyltartaric acid (DCTA) | 474 | Phenylpropanoic acid ester | V1, V3, V2 | 472.9 | 5.3, 5.4 | Neg. | [55] |
| Isoquercetin | 464 | Flavonoid | All | 463.0 | 5.6 | Neg. | [52] |
| Quercetin | 302 | Flavonoid | All | 303.0 | 5.4 | Pos. | [52] |
| Quercetin-3-O-glucose-6″-acetate | 506 | Flavonoid-3-O-glycoside | All | 505.0 | 5.8 | Neg. | [56] |
| Dicaffeoylquinic acid (DCQA) | 516 | Phenylpropanoic acid ester | V1, V3, V2 | 515.0 | 5.9, 5.8 | Neg. | [55] |
| 5-O-Caffeoylquinic acid (5-CQA) | 354 | Phenylpropanoic acid ester | V1, V2, V3 | 352.9 | 4.0, 3.9 | Neg. | [57] |
| Caffeic acid hexose | 342 | Phenylpropanoid derivative | V1 V3 | 340.9 340.8 | 5.7, 5.8 | Neg. | [58] |
| Quercetin 3-O-malonyl glucoside (QMG) | 550 | Flavonoid-3-O-glycoside | V1, V3, V2 | 551.1 | 5.6, 5.7 | Pos. | [52] |
| Luteolin 3′,4′-di-O-β-d-glucopyranoside | 610 | Flavonoid glycoside | V1, V2, V3 | 611.0 611.2 | 5.0, 5.1 | Pos. | [52] |
| Quercetin 3,4′-diglucoside | 626 | Flavonoid-3-O-glycoside | V1, V2, V3 | 627.1 627.1 627.2 | 4.1, 4.2, 4.2 | Pos. | [52] |
| Kaempferol malonyl glucoside (KMG) | 534 | Flavonoid-3-O-glycoside | V3 | 535.1 | 4.8 | Pos. | [59] |
| Metabolite | FC | Log2 (FC) | Raw p Value | −log10(p) |
|---|---|---|---|---|
| Butterhead green lettuce (Pazmanea RZ, V1) | ||||
| Luteolin-7-O-glucuronide | 5.8241 | 2.542 | 4.31 × 10−13 | 12.365 |
| Quercetin 3,4′-diglucoside | 30.015 | 4.9076 | 3.98 × 10−10 | 9.3999 |
| Quercetin-3-O-glucose-6′′-acetate | 12.783 | 3.6761 | 1.07 × 10−9 | 8.9706 |
| Isoquercetin | 7.0765 | 2.823 | 1.08 × 10−9 | 8.9659 |
| Quercetin | 11.674 | 3.5453 | 9.76 × 10−9 | 8.0105 |
| Caffeic acid hexose | 0.056472 | −4.1463 | 7.68 × 10−7 | 6.1145 |
| 5-O-Caffeoylquinic acid (5-CQA) | 5.5566 | 2.4742 | 1.40 × 10−6 | 5.8541 |
| Quercetin 3-O-malonylglucoside (QMG) | 6.5456 | 2.7105 | 3.21 × 10−5 | 4.493 |
| Luteolin 3′,4′-di-O-β-d-glucopyranoside | 3.3714 | 1.7533 | 0.00015003 | 3.8238 |
| Dicaffeoyltartaric acid (DCTA) | 1.7986 | 0.84687 | 0.00072512 | 3.1396 |
| Dicaffeoylquinic acid (DCQA) | 1.8121 | 0.85762 | 0.001202 | 2.9201 |
| Dihydrolactucopicrin | 2.9272 | 1.5495 | 0.0033116 | 2.48 |
| Lactucin | 2.7155 | 1.4412 | 0.028758 | 1.5412 |
| Lactucopicrin | 1.1981 | 0.26077 | 0.1786 | 0.74811 |
| Multi-leaf green lettuce (Hawking RZ, V2) | ||||
| Luteolin-7-O-glucuronide | 1.1586 | 0.21234 | 0.15066 | 0.82201 |
| Quercetin 3,4′-diglucoside | 2.1049 | 1.0738 | 1.04 × 10−5 | 4.9828 |
| Quercetin-3-O-glucose-6″-acetate | 1.8942 | 0.92157 | 0.0020218 | 2.6943 |
| Isoquercetin | 1.6636 | 0.73435 | 3.68 × 10−6 | 5.4341 |
| Quercetin | 1.7305 | 0.79122 | 7.44 × 10−5 | 4.1284 |
| 5-O-Caffeoylquinic acid (5-CQA) | 2.2622 | 1.1778 | 2.89 × 10−5 | 4.5399 |
| Quercetin 3-O-malonylglucoside (QMG) | 1.4393 | 0.52532 | 0.038325 | 1.4165 |
| Luteolin 3′,4′-di-O-β-d-glucopyranoside | 1.0201 | 0.028749 | 0.73936 | 0.13114 |
| Dicaffeoyltartaric acid (DCTA) | 1.1952 | 0.25728 | 0.13878 | 0.85767 |
| Dicaffeoylquinic acid (DCQA) | 1.5972 | 0.67556 | 0.00091261 | 3.0397 |
| Dihydrolactucopicrin | 1.9898 | 0.99263 | 0.0047081 | 2.3272 |
| Lactucopicrin | 2.4406 | 1.2872 | 0.0020288 | 2.6928 |
| Treatments | Antibacterial Activity % | DPPH IC50 (µg/mL) |
|---|---|---|
| V1/+S | 39.06 ± 7.8a | >200a |
| V1/−S | n.d | >200a |
| V2/+S | 39.06 ± 7.8a | >200a |
| V2/−S | n.d | >200a |
| V3/+S | 44.34 ± 5.41a | 60.53 ± 0.06b |
| V3/−S | 43.55 ± 7.34a | 62.83 ± 1.29b |
| Chloramphenicol | 98 at 3 μg/mL | |
| Ascorbic acid | 4.1 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, M.A.; Li, F.; Wenzel-Storjohann, A.; Sulieman, S.; Tasdemir, D.; Mühling, K.H. Comparative Metabolite Profile, Biological Activity and Overall Quality of Three Lettuce (Lactuca sativa L., Asteraceae) Cultivars in Response to Sulfur Nutrition. Pharmaceutics 2021, 13, 713. https://doi.org/10.3390/pharmaceutics13050713
Abdalla MA, Li F, Wenzel-Storjohann A, Sulieman S, Tasdemir D, Mühling KH. Comparative Metabolite Profile, Biological Activity and Overall Quality of Three Lettuce (Lactuca sativa L., Asteraceae) Cultivars in Response to Sulfur Nutrition. Pharmaceutics. 2021; 13(5):713. https://doi.org/10.3390/pharmaceutics13050713
Chicago/Turabian StyleAbdalla, Muna Ali, Fengjie Li, Arlette Wenzel-Storjohann, Saad Sulieman, Deniz Tasdemir, and Karl H. Mühling. 2021. "Comparative Metabolite Profile, Biological Activity and Overall Quality of Three Lettuce (Lactuca sativa L., Asteraceae) Cultivars in Response to Sulfur Nutrition" Pharmaceutics 13, no. 5: 713. https://doi.org/10.3390/pharmaceutics13050713
APA StyleAbdalla, M. A., Li, F., Wenzel-Storjohann, A., Sulieman, S., Tasdemir, D., & Mühling, K. H. (2021). Comparative Metabolite Profile, Biological Activity and Overall Quality of Three Lettuce (Lactuca sativa L., Asteraceae) Cultivars in Response to Sulfur Nutrition. Pharmaceutics, 13(5), 713. https://doi.org/10.3390/pharmaceutics13050713