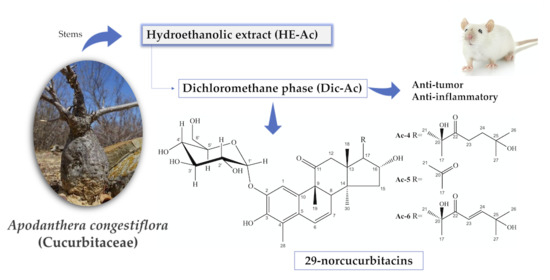
Anti-Tumor and Anti-Inflammatory Activity In Vivo of Apodanthera congestiflora Cogn. (Cucurbitaceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extaction and Liquid: Liquid Partition
2.3. Isolation of Chemical Compounds
2.4. Spectral Analysis
2.4.1. Cayaponoside C5b (Ac-1)
2.4.2. Cabenoside C (Ac-2)
2.4.3. Fevicordin C2 Glucoside (Ac-3)
2.5. Quantification of Cayaponoside C5b by Nuclear Magnetic Resonance (1H-NMR)
2.6. Toxicity in Human Erythrocytes
2.7. Biological Assays
2.7.1. Experimental Animals and Ethical Procedures
2.7.2. Acute Toxicity
2.7.3. Anti-Inflammatory Activity—Carrageenan-Induced Paw Edema
2.7.4. Anti-Tumor Activity in Ehrlich’s Carcinoma
2.7.5. Data Analysis
3. Results and Discussion
3.1. Caracterization of Mixture Ac-1, Ac-2 e Ac-3
3.2. Hemolytic Activity
3.3. Acute Toxicity
3.4. Anti-Inflammatory Activity
3.5. Anti-Tumor Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Z.; Chang, L. Cucurbitaceae. In Identification and Control of Common Weeds; Springer: Singapore, 2017; pp. 417–432. [Google Scholar]
- Gomes-Klein, V.L.; Hall, C.F.; Gil, A.S.B. Flora das cangas da Serra dos Carajás, Pará, Brasil: Cucurbitaceae. Rodriguésia 2016, 67, 1319–1327. [Google Scholar] [CrossRef]
- Silva, G.T.M.D.A.; Ribeiro, R.M.P.; Barros Júnior, A.P.; Silveira, L.M.; Ribeiro Júnior, P.M.; Albuquerque, N.R.C. Characterization of cucurbit production systems and disease prevalence in municipalities in Pernambuco. Rev. Caatinga 2016, 29, 774–779. [Google Scholar] [CrossRef]
- Méndez-Cuesta, C.A.; Campos, A.L.E.; Sánchez, D.S.; González, C.P.; Gutiérrez, S.P. Cytotoxic and antitumoral activities of compounds isolated from Cucurbitaceae plants. In Pharmacognosy—Medicinal Plants; IntechOpen: London, UK, 2018; pp. 1–18. [Google Scholar]
- Morales-vela, K.; Pérez-Sánchez, F.C.; Padrón, J.M.; Márquez-Fernándes, O. Antiproliferative activity of Cucurbitaceae species extracts from southeast of Mexico. Preprints 2019, 100, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Dan, C. Advances in research on the anticancer mechanism of the natural compound cucurbitacin from Cucurbitaceae plants: A review. Tradit. Med. Res. 2019, 4, 68–81. [Google Scholar] [CrossRef]
- Ali, M.S.; Mukherjee, S.; Makar, S.; Pal, G. Cucurbitacins a Vibrant Triterpenoid: A Review on its Anticancer Property. PharmaTutor 2019, 7, 43. [Google Scholar] [CrossRef]
- Ríos, J.L.; Escandell, J.M.; Recio, M.C. New insights into the bioactivity of cucurbitacins. Stud. Nat. Prod. Chem. 2005, 32, 429–469. [Google Scholar] [CrossRef]
- Kaushik, U.; Aeri, V.; Mir, S.R. Cucurbitacins—An insight into medicinal leads from nature. Pharmacogn. Rev. 2015, 9, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Madaleno, I.M. Plantas da medicina popular de São Luís, Brasil. Bol. Mus. Para. Emílio Goeldi. Ciênc. Hum. 2011, 6, 273–286. [Google Scholar] [CrossRef]
- Roque, A.A.; Loiola, M.I.B. Potencial de uso dos recursos vegetais em uma comunidade rural no semiárido potiguar. Rev. Caatinga 2013, 26, 88–98. [Google Scholar]
- Silva, C.G.; Marinho, M.G.V.; Lucena, M.F.A.; Costa, J.G.M. Levantamento etnobotânico de plantas medicinais em área de Caatinga na comunidade do ítio Nazaré, município de Milagres, Ceará, Brasil. Rev. Bras. Plantas Med. 2015, 17, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Pereira, H.N.; Guimarães, G.P.; de Melo Trovão, D.M.; da Silva Alves, H. Physicochemical characterization of the vegetal drug and nebulized extract of the roots from Apodanthera congestiflora Cogn. (Cucurbitaceae): An efficient experience in quality control of plant raw materials. J. Therm. Anal. Calorim. 2019, 137, 361–368. [Google Scholar] [CrossRef]
- Videres, L.C.C.A. Apodanthera Congestiflora e Myracrodruon Urundeuva: Investigação das Propriedades Biológicas em Preparações Brutas e Produtos Isolados. Ph.D. Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2017. [Google Scholar]
- Pinto, D.S.; Duarte, F.M.; Costa, J.I.V.; Almeida Filho, G.G.; Alves, H.S.; Chaves, M.C.O.; Pessoa, H.L.F.P. Antibacterial and Hemolytic Activities from Piper montealegreanum Yuncker (Piperaceae). Anti-Infect. Agents 2012, 10, 1–5. [Google Scholar] [CrossRef]
- OECD Guideline for Testing of Chemicals: Acute Oral Toxicity: Acute Toxic Class Method. [S.l.]. 2001. (OECD/OCDE, 423). Available online: https://www.oecd-ilibrary.org/environment/test-no-423-acute-oral-toxicity-acute-toxic-class-method_9789264071001-en (accessed on 17 December 2001).
- Stock, C.C.; Clark, D.A.; Philips, F.S.; Barclay, R.K.; Myron, S.A. Sarcoma 180 screening data. Cancer Res. 1960, 20, 193–381. [Google Scholar] [PubMed]
- Machon, Z.; Kuczynski, L.; Gieldamowski, J.; Wieczorek, Z.; Zimecki, M.; Blaszczyk, B.; Mordarski, M.; Wieczorek, J.; Fiszer-Maliszewska, L. Chemical and biological properties of 2-pyridyl-benzyl-carbinol. Arch. Immunol. Ther. Exp. 1981, 29, 217–233. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy, 4th ed.; Brooks/Cole: Belmont, CA, USA, 2012. [Google Scholar]
- Achenbach, H.; Waibel, R.; Hefter-Bübl, U.; Constenla, M.A. Constituents of Fevillea cordifolia: New Norcucurbitacin and Cucurbitacin Glycosides. J. Nat. Prod. 1993, 388, 1506–1519. [Google Scholar] [CrossRef]
- Nakano, K.; Kanai, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. Nor-cucurbitacin glucosides from Caputo nigri. Phytochemistry 1994, 37, 817–820. [Google Scholar] [CrossRef]
- Valente, L.M.M. Cucurbitacinas e suas principais características estruturais. Quim. Nova 2004, 27, 944–948. [Google Scholar] [CrossRef] [Green Version]
- Himeno, E.; Nagao, T.; Honda, J.; Okabe, H.; Irino, N.; Nakasumi, T. Studies on the Constituents of the Root of Cayaponia tayuya (VELL.) COGN. III. Structures of Cayaponosides, 29-Nor-1,2,3,4,5,10-hexadehydrocucurbit-6-ene Glucosides. Chem. Pharm. Bull. 1994, 42, 2370–2372. [Google Scholar] [CrossRef] [Green Version]
- Achenbach, H.; Hefter-Bübl, U.; Constenla, M.A. Fevicordin A and fevicordin A glucoside, novel norcucurbitacins from Fevillea cordifolia. J. Chem. Soc. Chem. Commun. 1987, 441, 441–442. [Google Scholar] [CrossRef]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordel, G.A.; Qiuz, S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef]
- Silvestre, G.F.G. Estudo Fitoquímico e Farmacológico de Apodanthera congestiflora Cogn.: Uma Cucurbitaceae da Caatinga Brasileira. Master’s Thesis, Universidade Estadual da Paraíba, Campina Grande, Brazil, 2020. [Google Scholar]
- Tavares, R.L.; De Vasconcelos, M.H.A.; da Veiga Dutra, M.L.; D’Oliveira, A.B.; Lima, M.D.S.; da Silva Stiebbe Salvadori, M.G.; de Alencar Pereira, R.; Alves, A.F.; do Nascimento, Y.M.; Tavares, J.F.; et al. Mucuna pruriens Administration Minimizes Neuroinflammation and Shows Anxiolytic, Antidepressant and Slimming Effects in Obese Rats. Molecules 2020, 25, 5559. [Google Scholar] [CrossRef] [PubMed]
- Simões, C.M.O.; Schenkel, E.P.; Gosman, G.; Mello, J.C.P.; Mentz, L.A.; Petrovick, P.R. Farmacognosia: Da Planta ao Medicamento; Editora da UFSC: Florianópolis, Brazil, 2007. [Google Scholar]
- Pereira, H.N. Estudo Fitoquímico e Atividade Biológica das Raízes de Apodanthera congestiflora Cogn. (Cucurbitaceae). Master’s Thesis, Universidade Estadual da Paraíba, Campina Grande, Brazil, 2017. [Google Scholar]
- Oga, S.; Camargo, M.M.D.A.; Batistuzzo, J.A.D.O. Fundamentos da Toxicologia, 3rd ed.; Atheneu: São Paulo, Brazil, 2008. [Google Scholar]
- Guyton, A.C.; Hall, J.E. Tratado de Fisiologia Médica, 13th ed.; Elsevier: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Schons, S.V.; Kommers, G.D.; Pereira, G.M.; Raffi, M.B.; Schild, A.L. Estudo histológico, imuno-histoquímico e ultra-estrutural das lesões induzidas experimentalmente por Ramaria flavo-brunnescens (Clavariaceae) em bovinos. Pesq. Vet. Bras. 2007, 27, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Peters, R.R.; Krepsky, P.B.; Siqueira-Junior, J.M.; Rocha, J.C.S.; Bezerra, M.M.; Ribeiro, R.A.; Brum-Fernandes, A.J.; Farias, M.R.; Rocha, F.A.C.; Ribeiro-Do-Valle, R.M. Nitric oxide and cyclooxygenase may participate in the analgesic and anti-inflammatory effect of the cucurbitacins fraction from Wilbrandia ebracteata. Life Sci. 2003, 73, 2185–2197. [Google Scholar] [CrossRef]
- Ayyad, S.-E.N.; Abdel-Lateff, A.; Alarif, W.M.; Patacchioli, F.R.; Badria, F.A.; Ezmirly, S.T. In vitro and in vivo study of cucurbitacins-type triterpene glucoside from Citrullus colocynthis growing in Saudi Arabia against hepatocellular carcinoma. Environ. Toxicol. Pharmacol. 2012, 33, 245–251. [Google Scholar] [CrossRef] [PubMed]




| Cayaponoside C5b | Cabenoside C | Fevicordin C2 Glucoside | ||||
|---|---|---|---|---|---|---|
| Nº | δC, | δH (J in Hz) | δC, | δH (J in Hz) | δC, | δH (J in Hz) |
| 1 | 112.8 | 6.60 (s) | 112.7 | 6.58 (s) | 112.8 | 6.58 (s) |
| 2 | 145.2 | 144.9 | 145.1 | |||
| 3 | 144.9 | 145.1 | 145.1 | |||
| 4 | 122.3 | 122.3 | 122.4 | |||
| 5 | 129.3 | 127.8 | 129.4 | |||
| 6 | 125.6 | 6.87 (d, 10) | 125.6 | 6.88 (d, 9.9) | 125.6 | 6.88 (d, 9.9) |
| 7 | 127.2 | 5.80 (dd, 6.2, 10) | 126.9 | 5.82 (dd, 6.2, 10) | 127.3 | 5.82 (dd, 6, 2, 10) |
| 8 | 47.4 | 2.56 (m) | 47.6 | 2.54 (m) | 47.5 | |
| 9 | 51.7 | 50.5 | 50.1 | |||
| 10 | 129.4 | 129.2 | 129.3 | |||
| 11 | 213.8 | 212.7 | 213.8 | |||
| 12 | 51.1 | 2.71 (d, 14.9) 3.05 (d, 14.9) | 49.7 | 2.87 (m) 3.17 (m) | 50.9 | 2.87 (m) 2.67 (m) |
| 13 | 50.0 | 49.0 | 50.5 | |||
| 14 | 48.7 | 49.5 | 50.9 | |||
| 15 | 44.5 | 1.33 (sl) 2.05 (m) | 44.2 | 2.21 (sl) | 48.6 | 1.33 (sl) |
| 16 | 71.2 | 4.34 (t, 7.5) | 71.3 | 4.66 (m) | 71.4 | 4.48 (m) |
| 17 | 58.7 | 2.53 (m) | 67.7 | 3.07 (m) | 58.8 | 2.66 (d, 7.9) |
| 18 | 17.9 | 0.95 (s) | 19.7 | 0.95 (s) | 20.0 | 0.95 (s) |
| 19 | 26.7 | 1.20 (s) | 26.5 | 1.38 (s) | 29.8 | 1.20 (s) |
| 20 | 79.8 | 208.6 | 79.9 | |||
| 21 | 25.2 | 1.38 (s) | 31.7 | 2.13 (s) | 26.0 | 1.37 (s) |
| 22 | 215.6 | 203.6 | ||||
| 23 | 32.1 CH2 | 2.86 (m) | 120.6 CH | 6.81 (d, 15.4) | ||
| 24 | 37.9 CH2 | 1.69 (t, 7.9) | 155.3 CH | 6.94 (d, 15.3) | ||
| 25 | 79.9 | 69.7 | ||||
| 26 | 29.4 | 1.13 (s) | 30.4 | 1.26 (s) | ||
| 27 | 29.4 | 1.12 (s) | 29.7 | 1.27 (s) | ||
| 28 | 11.2 | 2.21 (s) | 11.2 | 2.22 (s) | 11.2 | 2.22 (s) |
| 30 | 20.2 | 1.01 (s) | 17.8 | 1.01 (s) | 19.7 | 1.01 (s) |
| 1′ | 104.5 | 4.65 (d, 7.4) | 104.6 | 4.65 (d, 7.4) | 104.1 | 4.65 (d, 7.4) |
| 2′ | 74.3 | 3.59–3.40 (m) | 74.5 | 3.59–3.40 (m) | 74.6 | 3.59–3.40 (m) |
| 3′ | 77.2 | 3.59–3.40 (m) | 77.2 | 3.59–3.40 (m) | 77.2 | 3.59–3.40 (m) |
| 4′ | 70.7 | 3.59–3.40 (m) | 70.6 | 3.59–3.40 (m) | 70.3 | 3.59–3.40 (m) |
| 5′ | 77.7 | 3.39 (m) | 77.7 | 3.39 (m) | 77.7 | 3.39 (m) |
| 6′ | 62.0 | 3.92 (dd, 2.8 and 12) 3.80 (dd, 4.5 and 12) | 62.3 | 3.92 (dd, 2.8 and 12) 3.80 (dd, 4.5 and 12) | 62.3 | 3.92 (dd, 2.8 and 12) 3.80 (dd, 4.5 and 12) |
| Group | Weight (g) | Water Consumption by Animal (mL) | Feed Consumption by Animal (g) | |
|---|---|---|---|---|
| Initial | Final | |||
| Control | 28.2 ± 1.58 | 36.0 ± 1.01 | 9.97 ± 0.15 | 7.89 ± 0.25 |
| Treated with Dic-Ac | 26.3 ± 1.81 | 30.6 ± 1.47 * | 12.84 ± 0.87 * | 6.49 ± 0.98 |
| Group | Organ Index | ||||
|---|---|---|---|---|---|
| Lungs | Heart | Kidney | Liver | Spleen | |
| Control | 0.15 ± 0.01 | 0.13 ± 0.01 | 0.42 ± 0.03 | 1.66 ± 0.04 | 0.22 ± 0.07 |
| Treated with Dic-Ac | 0.14 ± 0.02 | 0.12 ± 0.02 | 0.46 ± 0.01 | 2.47 ± 0.06 * | 0.24 ± 0.03 |
| Test | Control | Treated with Dic-Ac | |
|---|---|---|---|
| Biochemical | Urea (mg dL−1) | 16.43 ± 2.03 | 18.1 ± 1.33 |
| Creatinine (mg dL−1) | 0.41 ± 0.09 | 0.42 ± 0.03 | |
| AST (U L−1) | 10.93 ± 4.30 | 12.42 ± 5.61 | |
| ALT (U L−1) | 16.57 ± 9.50 | 19.28 ± 8.65 | |
| Alkaline phosphatase (U L−1) | 26.42 ± 6.71 | 31.36 ± 8.24 | |
| Total protein (g dL−1) | 3.67 ± 1.12 | 4.63 ± 0.62 | |
| Albumin (g dL−1) | 1.44 ± 0.26 | 1.68 ± 0.11 | |
| Glucose (mg dL−1) | 69.00 ± 1.73 | 49.00 ± 2.33 * | |
| Total cholesterol (mg dL−1) | 149.70 ± 10.80 | 116.30 ± 3.28 * | |
| Triglycerides (mg dL−1) | 47.33 ± 7.48 | 43.67 ± 3.61 | |
| Total bilirubin (mg dL−1) | 0.62 ± 0.17 | 0.61± 0.12 | |
| Gamma-GT (mg dL−1) | 20.36 ± 4.29 | 19.37 ± 3.84 | |
| Hematological | Red blood cells count (mm3) | 5.29 ± 0.33 | 5,74 ± 0,41 |
| Hemoglobin concentration (g dL−1) | 15.14 ± 0.03 | 14,63 ± 0.05 | |
| Hematocrit (%) | 46.00 ± 1.31 | 44.00 ± 1.46 | |
| MCV (fL) | 45.53 ± 0.05 | 44.24 ± 0.07 | |
| MCH (pg) | 16.54 ± 0.07 | 15.39 ± 0.01 | |
| MCHC (%) | 35.67 ± 0.32 | 34.93 ± 0.28 | |
| Platelet count (mm3) | 162.08 ± 0.26 | 148.68 ± 0.21 | |
| White blood cells count (mm3) | 6.00 ± 0.48 | 4.50 ± 0.29 | |
| Segmented neutrophils (%) | 40 | 45 | |
| Eosinophils (%) | 01 | 04 | |
| Lymphocytes (%) | 57 | 50 | |
| Monocytes (%) | 02 | 01 |
| Group | Oral Dose | Inhibition (%) | ||||
|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | ||
| Negative control | ||||||
| Dexamethasone | 10 mg kg−1 | 70.2 | 81.8 | 88.5 | 83.6 | 79.5 |
| Dic-Ac | 30 mg kg−1 | 37.5 | 48.4 | 49.7 | 51.2 | 64.3 |
| Dic-Ac | 15 mg kg−1 | 27.6 | 42.8 | 52.4 | 51.6 | 57.3 |
| Dic-Ac | 7.5 mg kg−1 | 58.7 | 66.6 | 62.7 | 61.3 | 60.9 |
| Group | Oral Dose |
Average Weight (g) |
Inhibition Index (TW%) |
|---|---|---|---|
| Negative control | 2.25 g | 0.00% | |
| Standard Cisplatin | 2.5 mg kg−1 | 1.15 g | 62.01% |
| Dic-Ac | 30 mg kg −1 | 0.78 g | 76.05% |
| Dic-Ac | 15 mg kg −1 | 0.70 g | 78.46% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestre, G.F.G.; Lucena, R.P.; Oliveira, G.D.; Pereira, H.N.; Dias, J.A.B.; Souza, I.A.; Alves, H.S. Anti-Tumor and Anti-Inflammatory Activity In Vivo of Apodanthera congestiflora Cogn. (Cucurbitaceae). Pharmaceutics 2021, 13, 743. https://doi.org/10.3390/pharmaceutics13050743
Silvestre GFG, Lucena RP, Oliveira GD, Pereira HN, Dias JAB, Souza IA, Alves HS. Anti-Tumor and Anti-Inflammatory Activity In Vivo of Apodanthera congestiflora Cogn. (Cucurbitaceae). Pharmaceutics. 2021; 13(5):743. https://doi.org/10.3390/pharmaceutics13050743
Chicago/Turabian StyleSilvestre, Geovana F. G., Renally P. Lucena, Genil D. Oliveira, Helimarcos N. Pereira, Jhonatta A. B. Dias, Ivone A. Souza, and Harley S. Alves. 2021. "Anti-Tumor and Anti-Inflammatory Activity In Vivo of Apodanthera congestiflora Cogn. (Cucurbitaceae)" Pharmaceutics 13, no. 5: 743. https://doi.org/10.3390/pharmaceutics13050743
APA StyleSilvestre, G. F. G., Lucena, R. P., Oliveira, G. D., Pereira, H. N., Dias, J. A. B., Souza, I. A., & Alves, H. S. (2021). Anti-Tumor and Anti-Inflammatory Activity In Vivo of Apodanthera congestiflora Cogn. (Cucurbitaceae). Pharmaceutics, 13(5), 743. https://doi.org/10.3390/pharmaceutics13050743