Polymeric Micelles for the Enhanced Deposition of Hydrophobic Drugs into Ocular Tissues, without Plasma Exposure
Abstract
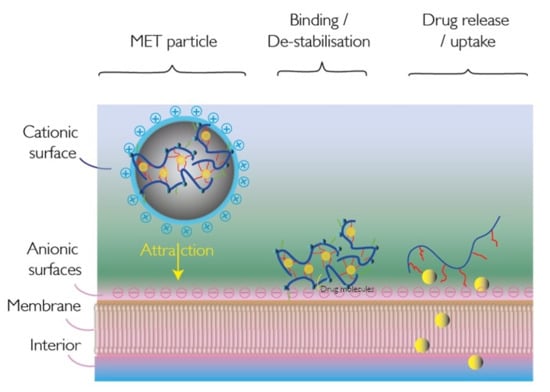
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation Preparation
2.2.1. Method I—High Pressure Homogenization (Non-Sterile with Glycerol)
2.2.2. Method II—High Pressure Homogenization (Sterile with Glycerol)
2.2.3. Method III—High Pressure Homogenization (Non-Sterile-Phosphate Buffered Saline)
2.2.4. Method IV—Probe Sonication (Non-Sterile with Glycerol)
2.3. Formulation Characterisation
2.3.1. Drug Content
2.3.2. MET Content
2.3.3. Osmolarity
2.3.4. Electron Microscopy
2.3.5. Particle Size
2.3.6. Zeta Potential
2.4. Formulation Stability
2.5. Ocular Pharmacokinetics
Animal Ocular Biodistribution Studies
2.6. Sample Analysis
2.6.1. Preparation of Standards
2.6.2. Tissue Processing
2.6.3. LC-MS/MS Instrumentation
2.6.4. Chromatography Conditions
2.6.5. Mass Spectrometer Conditions
2.6.6. Quantification
2.6.7. Pharmacokinetic Analysis
2.7. Statistical Analysis for Pharmacokinetics Studies
2.8. Ocular Tolerability of the MET Polymer
3. Results
3.1. Formulation and Stability Studies
3.2. Stability Studies
3.3. Ocular Tolerability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mochizuki, H.; Yamada, M.; Hato, S.; Nishida, T. Fluorophotometric measurement of the precorneal residence time of topically applied hyaluronic acid. Br. J. Ophthalmol. 2007, 92, 108–111. [Google Scholar] [CrossRef] [Green Version]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Qu, X.; Khutoryanskiy, V.V.; Stewart, A.; Rahman, S.; Papahadjopoulos-Sternberg, B.; Dufes, C.; McCarthy, D.; Wilson, C.G.; Lyons, R.; Carter, K.C.; et al. Carbohydrate-Based Micelle Clusters Which Enhance Hydrophobic Drug Bioavailability by Up to Order of Magnitude. Biomacromolecules 2006, 7, 3452–3459. [Google Scholar] [CrossRef] [PubMed]
- Molpeceres, J.; Guzman, M.; Bustamante, P.; Aberturas, M.D.R. Exothermic-endothermic heat of solution shift of cyclosporin A related to poloxamer 188 behaviour in aqueous solution. Int. J. Pharm. 1996, 130, 75–81. [Google Scholar] [CrossRef]
- Mantelli, F.; Massaro-Giordano, M.; Macchi, I.; Lambiase, A.; Bonini, S. The cellular mechanisms of dry eye: From pathogenesis to treatment. J. Cell. Physiol. 2013, 228, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Q.; Wei, R.L. Topical Cyclosporine A in the Treatment of Dry Eye. Cornea 2014, 33, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Tzioufas, A.G.; Stone, J.H.; Sisó, A.; Bosch, X. Treatment of Primary Sjögren Syndrome. JAMA 2010, 304, 452–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency. Ikervis Assessment Report. 2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002066/WC500186592.pdf (accessed on 12 December 2017).
- Rao, S.N. Topical Cyclosporine 0.05% for the Prevention of Dry Eye Disease Progression. J. Ocul. Pharmacol. Ther. 2010, 26, 157–164. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Ikervis. 2015. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002066/smops/Positive/human_smop_000783.jsp&mid=WC002060b002001ac058001d002127 (accessed on 12 December 2017).
- Dastjerdi, M.H.; Hamrah, P.; Dana, R. High-frequency Topical Cyclosporine 0.05% in the Treatment of Severe Dry Eye Refractory to Twice-daily Regimen. Cornea 2009, 28, 1091–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, K.H.-N.; Chen, L.J.; Rong, S.S.; Pang, C.P.; Young, A.L. Topical Cyclosporine in the Treatment of Allergic Conjunctivitis. Ophthalmology 2013, 120, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alcalde, J.; Laiz, R.M.; Buenaga, R.F.; Fernández, G.R. Topical cyclosporine for atopic keratoconjunctivitis. Cochrane Database Syst. Rev. 2012, 10, CD009078. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Scoper, S.V.; Samudre, S. Topical Loteprednol Pretreatment Reduces Cyclosporine Stinging in Chronic Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2011, 27, 23–27. [Google Scholar] [CrossRef]
- USFDA. Application Number 21-023 Medical Reviews. 2002. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/2021-2023_Restasis_Medr.PDF (accessed on 12 December 2017).
- Siew, A.; Le, H.; Thiovolet, M.; Gellert, P.; Schatzlein, A.; Uchegbu, I. Enhanced Oral Absorption of Hydrophobic and Hydrophilic Drugs Using Quaternary Ammonium Palmitoyl Glycol Chitosan Nanoparticles. Mol. Pharm. 2011, 9, 14–28. [Google Scholar] [CrossRef]
- Chooi, K.W.; Carlos, M.I.S.; Soundararajan, R.; Gaisford, S.; Arifin, N.; Schätzlein, A.G.; Uchegbu, I.F. Physical Characterisation and Long-Term Stability Studies on Quaternary Ammonium Palmitoyl Glycol Chitosan (GCPQ)—A New Drug Delivery Polymer. J. Pharm. Sci. 2014, 103, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Odunze, U.; O’Brien, F.; Godfrey, L.; Schatzlein, A.; Uchegbu, I. Unusual Enthalpy Driven Self Assembly at Room Temperature with Chitosan Amphiphiles. Pharm. Nanotechnol. 2019, 7, 57–71. [Google Scholar] [CrossRef]
- Cheng, W.P.; Gray, A.I.; Tetley, L.; Hang, T.L.B.; Schätzlein, A.A.G.; Uchegbu, I.F. Polyelectrolyte Nanoparticles with High Drug Loading Enhance the Oral Uptake of Hydrophobic Compounds. Biomacromolecules 2006, 7, 1509–1520. [Google Scholar] [CrossRef]
- Badr, M.Y.; Abdulrahman, N.S.; Schatzlein, A.G.; Uchegbu, I.F. A polymeric aqueous tacrolimus formulation for topical ocular delivery. Int. J. Pharm. 2021, 599, 120364. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef] [PubMed]
- British Pharmacopoeia. British Pharmacopoeia Online Volume I and II; The Stationary Office: London, UK, 2012; Available online: https://www.pharmacopoeia.com (accessed on 12 June 2015).
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy, 4th ed.; McMillan Press, Ltd.: London, UK, 2006; p. 564. [Google Scholar]
- Bunjes, D.; Hardt, C.; Röllinghoff, M.; Wagner, H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur. J. Immunol. 1981, 11, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Daull, P.; Lallemand, F.; Philips, B.; Lambert, G.; Buggage, R.; Garrigue, J.-S. Distribution of Cyclosporine A in Ocular Tissues After Topical Administration of Cyclosporine A Cationic Emulsions to Pigmented Rabbits. Cornea 2013, 32, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Scherer, D.; Alvarez-Gonzalez, E.; Pettigrew, T. EyeSol: A Novel Topical Ocular Drug Delivery System for Poorly Soluble Drugs. Drug Development and Delivery 2013, January 2013. Available online: http://mx1.specialtypharma.com/Main/Back-Issues/EyeSol-a-Novel-Topical-Ocular-Drug-Delivery-System-137.aspx (accessed on 14 January 2017).
- Cholkar, K.; Gilger, B.C.; Mitra, A.K. Topical, Aqueous, Clear Cyclosporine Formulation Design for Anterior and Posterior Ocular Delivery. Transl. Vis. Sci. Technol. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Formulation Name | MET (% w/v) | Cyclosporine A (% w/v) | Diluent | Method of Preparation |
|---|---|---|---|---|
| NM133 0.01% A * | 0.75 | 0.01 | Glycerol (3.1% w/v), pH = 7.4 | Method I |
| NM133 0.05% A | 0.75 | 0.05 | Glycerol (3.1%w/v), pH = 7.4 | Method I |
| NM133 0.01% B | 0.75 | 0.01 | Glycerol (2.7% w/v), pH = 7.0 | Method II |
| NM133 0.02% A | 0.75 | 0.02 | Glycerol (2.7% w/v), pH = 7.0 | Method II |
| NM133 0.05% B | 0.75 | 0.05 | Glycerol (2.7% w/v), pH = 7.0 | Method II |
| NM133 0.08% A | 0.75 | 0.08 | Glycerol (1.0% w/v) | Method IV |
| NM133 0.08% B | 0.75 | 0.08 | Glycerol (1.0% w/v) | Method IV |
| NM133 0.1% A | 0.75 | 0.1 | Glycerol (1.0% w/v) | Method IV |
| NM0133 0.05% C | 0.375 | 0.05 | Phosphate buffered saline (PBS, pH = 7.4) | Method III |
| NM0133 0.08% C | 0.60 | 0.08 | PBS (pH = 7.4) | Method III |
| NM0133 0.08% D | 0.75 | 0.08 | PBS (pH = 7.4) | Method III |
| NM0133 0.1% B | 0.75 | 0.1 | PBS (pH = 7.4) | Method III |
| Formulation Strength | Nominal MET Concentration (mg mL−1) | CsA Conc. (mg mL−1) | z-Average Mean Particle Size (nm)/PDI | Zeta Potential (mV) | Viscosity mPa.s | pH | Osmolarity (mOsm L−1) | Disperse Phase |
|---|---|---|---|---|---|---|---|---|
| 0.01% B | 7.5 | 0.094 ± 0.001 | 39 ± 1.6/ 0.435 ± 0.004 | +34 ± 0.7 | 1.338 ± 0.040 | 6.90 ± 0.0 | 313 ± 9 | 2.7% glycerol in water, pH adjusted to pH = 7 |
| 0.02% A | 7.5 | 0.201 ± 0.007 | 37 + 0.7/ 0.414 ± 0.022 | +26 ± 1.1 | 1.230 ± 0.040 | 7.0 ± 0.0 | 320 ± 3 | 2.7% glycerol in water, pH adjusted to pH = 7 |
| 0.05% B | 7.5 | 0.510 ± 0.0004 | 36 ± 1.2/ 0.395 ± 0.020 | +33 ± 1.9 | 1.303 ± 0.052 | 6.9 ± 0.0 | 323 ± 9 | 2.7% glycerol in water, pH adjusted to pH = 7 |
| 0.05% C | 3.75 | 0.497 ± 0.0062 | 835 ± 43/ polydisperse (PDI > 0.6) | ND | ND | 6.8 ± 0.3 | 311 ± 1 | PBS, pH = 7 |
| 0.08% C | 7.5 | 0.782 ± 0.0088 | 255 ± 96/ polydisperse (PDI > 0.6) | ND | ND | ND | PBS, pH = 7 | |
| 0.1% B | 7.5 | 1.02 ± 0.0098 | 806 ± 245/ polydisperse (PDI > 0.6) | ND | ND | 7.0 ± 0.2 | 304 ± 1 | PBS, pH = 7 |
| Formulation Strength | Day 0 | Day 28 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CsA Conc. (mg mL−1) | pH | Osmolarity (mOsm L−1) | Viscosity (mPa.s) | Zeta Potential (mV) | z-Average Mean Particle Size (nm)/PDI | CsA Conc. (mg mL−1) | pH | Osmolarity (mOsm L−1) | Viscosity (mPa.s) | Zeta Potential (mV) | z-Average Mean Size (nm)/PDI | |
| 0.01% B | 0.094 ± 0.001 | 6.93 ± 0.06 | 313 ± 9 | 1.338 ± 0.040 | +34 ± 0.7 | 39 ± 1.6/ 0.435 ± 0.004 | 0.106 ± 0.008 | ND | 328 ± 8 * | 1.210 ± 0.006 * | +20 ± 1.4 * | 28 ± 1.7/ 0.408 ± 0.007 |
| 0.02% A | 0.201 ± 0.007 | 6.93 ± 0.06 | 320 ± 3 | 1.23 ± 0.004 | +26 ± 1.1 | 37 + 0.7/ 0.414 ± 0.022 | 0.228 ± 0.018 * | ND | 383 ± 9 * | 1.207 ± 0.005 | +20 ± 1.5 * | 28 ± 1.0/ 0.411 ± 0.0134 |
| 0.05% B | 0.510 ± 0.004 | 6.93 ± 0.06 | 323 ± 9 | 1.303 ± 0.052 | +33 ± 1.9 | 36 ± 1.2/ 0.395 ± 0.020 | 0.533 ± 0.031 | ND | 349 ± 7 * | 1.339 ± 0.020 | +41 ± 3.2 * | 24 ± 0.6/ 0.398 ± 0.005 |
| Formulation Strength | Day 0 | Day 28 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CsA Conc. (mg mL−1) | pH | Osmolarity (mOsm L−1) | Viscosity (mPa.s) | Zeta Potential (mV) | z-Average Mean Particle Size (nm)/PDI | CsA Conc. (mg mL−1) | pH | Osmolarity (mOsm L−1) | Viscosity (mPa.s) | Zeta Potential (mV) | z-Average Mean Particle Size (nm)/PDI | |
| 0.01% B | 0.094 ± 0.001 | 6.9 ± 0.0 | 313 ± 9 | 1.338 ± 0.040 | +34 ± 0.7 | 39 ± 1.6/ 0.435 ± 0.004 | 0.100 ± 0.003 * | ND | 324 ± 11 | 1.215 ± 0.003 * | +23 ± 7 | 32 ± 2.6/ 0.380 ± 0.038 |
| 0.02% A | 0.201 ± 0.007 | 7.0 ± 0.0 | 320 ± 3 | 1.230 ± 0.004 | +26 ± 1.1 | 37 + 0.7/ 0.414 ± 0.022 | 0.203 ± 0.008 | ND | 339 ± 4 * | 1.208 ± 0.002 | +26 ± 2.6 | 29 ± 0.5/ 0.400 ± 0.016 |
| 0.05% B | 0.510 ± 0.004 | 6.9 ± 0.0 | 323 ± 9 | 1.303 ± 0.052 | +33 ± 1.9 | 36 ± 1.2/ 0.395 ± 0.020 | 0.506 ± 0.022 | ND | 330 ± 1 | 1.358 ± 0.005 * | +31 ± 3.8 | 28 ± 0.5/ 0.317 ± 0.018 |
| Formulation Strength | Day 0 | Day 28 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CsA Conc. (mg mL−1) | pH | Osmolarity (mOsm L−1) | Viscosity (mPa.s) | Zeta Potential (mV) | z-Average Mean Particle Size (nm)/PDI | CsA Conc. (mg mL−1) | pH | Osmolarity (mOsm L−1) | Viscosity (mPa.s) | Zeta Potential (mV) | z-Average Mean Particle Size (nm)/PDI | |
| 0.01% B | 0.094 ± 0.001 | 6.9 ± 0.0 | 313 ± 9 | 1.338 ± 0.040 | +34 ± 0.7 | 39 ± 1.6/ 0.435 ± 0.004 | 0.097 ± 0.005 | ND | 319 ± 13 | 1.234 ± 0.032 * | +35 ± 0.1 | 33 ± 1.9/0.387 ± 0.062 |
| 0.02% A | 0.201 ± 0.007 | 7.0 ± 0.0 | 320 ± 3 | 1.230 ± 0.004 | +26 ± 1.1 | 37 + 0.7/ 0.414 ± 0.022 | 0.202 ± 0.011 | ND | 331 ± 1 * | 1.234 ± 0.007 | +37 ± 0.9 * | 33 ± 0.4/0.409 ± 0.004 |
| 0.05% B | 0.510 ± 0.004 | 6.9 ± 0.0 | 323 ± 9 | 1.303 ± 0.052 | +33 ± 1.9 | 36 ± 1.2/ 0.395 ± 0.020 | 0.502 ± 0.024 | ND | 324 ± 5 | 1.209 ± 0.015* | +36 ± 3.2 | 30 ± 0.3/0.385 ± 0.041 |
| Storage Days | Cyclosporine A Concentration (Mean ± s.d., mg mL−1) | Z-Average Mean Particle Size (nm) * | Storage Temperature |
|---|---|---|---|
| 0 | 0.782 ± 0.088 | 255 ± 96 | Not Applicable |
| 1–2 | 0.725 ± 0.026 | 336 ± 136 | −20 °C |
| 3–4 | 0.907 ± 0.108 | 332 ± 97 | 4 °C |
| 5–6 | 0.724 ± 0.070 | 386 ± 96 | 40 °C |
| 7–8 | 0.863 ± 0.113 | 381 ± 134 | −20 °C |
| 9–10 | 0.820 ± 0.068 | 325 ± 10 | 4 °C |
| 11–12 | 0.773 ± 0.058 | 294 ± 37 | 40 °C |
| 13–14 | 0.787 ± 0.107 | 332 ± 65 | −20 °C |
| 15–16 | 0.697 ± 0.164 | 279 ± 73 | 4 °C |
| 17–18 | 0.834 ± 0.067 | 374 ± 118 | 40 °C |
| Formulation | Number of Colony Forming Units | ||
|---|---|---|---|
| Freshly Prepared | 24 h after Sterile Filtration | 5 Days after Sterile Filtration | |
| NM133 0.05%A Sample 1 | 1 | 0 | 1 |
| NM133 0.05%A Sample 2 | 0 | 0 | 0 |
| NM133 0.05%A Sample 3 | 3 | 0 | 0 |
| MET 0.75% Sample 1 | 0 | 0 | 0 |
| MET 0.75% Sample 2 | 0 | 0 | 0 |
| Formulation | Cyclosporine A Topical Ocular Dose (μg) | AUC(0–24) (ng.h/g) | Cmax (ng mL−1) | ||||
|---|---|---|---|---|---|---|---|
| Cornea | Conjunctiva | Sclera | Cornea | Conjunctiva | Sclera | ||
| NM133 0.05% A | 12.5 | 25,780 | 12,046 | 5879 | 1546 | 3864 | 627 |
| NM133 0.01% A | 2.5 | 8024 | 3988 | 1372 | 410 | 703 | 146 |
| Restasis 0.05% | 12.5 | 4726 | 4813 | 1729 | 216 | 608 | 101 |
| Formulation | Blink Rate per Minute |
|---|---|
| NM0133 0.05% A | 5.3 ± 2.76 |
| NM0133 0.01% A | 4.7 ± 2.25 |
| Restasis | 6.1 ± 2.56 |
| Formulation | Total Ocular Dose (μg) | Cornea Cmax (ng g−1) | Conjunctiva Cmax (ng g−1) * | Dose Strength (% w/v) | Dose Volume (μL) | Calculated Corneal Cmax per μg Dosed | Calculated Conjunctival Cmax per μg Dosed | Reference |
|---|---|---|---|---|---|---|---|---|
| NOVA22007 | 50 | 2691 | 1914 | 0.1 | 50 | 54 | 38 | [26] |
| NOVA22007 | 25 | 1372 | 696 | 0.05 | 50 | 55 | 29 | [26] |
| Comparative Restasis Data from the Cornea 2013 Study | 25 | 748 | 848 | 0.05 | 50 | 30 | 34 | [26] |
| Cequa * (one hour time point) | 35 | 828 | 1417 | 0.1 | 35 | 24 | 40 | [28] |
| NM133 | 12.5 | 1546 | 3864 | 0.05 | 25 | 124 | 309 | Current study |
| NM133 | 2.5 | 409 | 703 | 0.01 | 25 | 164 | 281 | Current study |
| Comparative Restasis Data in Nanomerics’ Studies | 12.5 | 216 | 608 | 0.05 | 25 | 17 | 49 | Current study |
| Cyclasol | 100 | 1326 | Not given | Not given | Not given | 13 | N/A | [27] |
| Oil In water emulsion tested by Novaliq | 100 | 633 | Not given | Not given | Not given | 6 | N/A | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchegbu, I.F.; Breznikar, J.; Zaffalon, A.; Odunze, U.; Schätzlein, A.G. Polymeric Micelles for the Enhanced Deposition of Hydrophobic Drugs into Ocular Tissues, without Plasma Exposure. Pharmaceutics 2021, 13, 744. https://doi.org/10.3390/pharmaceutics13050744
Uchegbu IF, Breznikar J, Zaffalon A, Odunze U, Schätzlein AG. Polymeric Micelles for the Enhanced Deposition of Hydrophobic Drugs into Ocular Tissues, without Plasma Exposure. Pharmaceutics. 2021; 13(5):744. https://doi.org/10.3390/pharmaceutics13050744
Chicago/Turabian StyleUchegbu, Ijeoma F., Jan Breznikar, Alessandra Zaffalon, Uche Odunze, and Andreas G. Schätzlein. 2021. "Polymeric Micelles for the Enhanced Deposition of Hydrophobic Drugs into Ocular Tissues, without Plasma Exposure" Pharmaceutics 13, no. 5: 744. https://doi.org/10.3390/pharmaceutics13050744
APA StyleUchegbu, I. F., Breznikar, J., Zaffalon, A., Odunze, U., & Schätzlein, A. G. (2021). Polymeric Micelles for the Enhanced Deposition of Hydrophobic Drugs into Ocular Tissues, without Plasma Exposure. Pharmaceutics, 13(5), 744. https://doi.org/10.3390/pharmaceutics13050744