The Chronotopic™ System for Pulsatile and Colonic Delivery of Active Molecules in the Era of Precision Medicine: Feasibility by 3D Printing via Fused Deposition Modeling (FDM)
Abstract
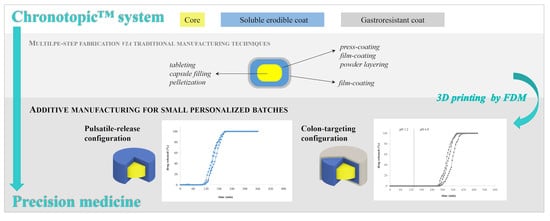
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extrusion of Filaments
2.2.2. Printing of Prototypes
2.2.3. Characterization of Prototypes
2.2.4. Mechanical Characterization
3. Results and Discussion
3.1. Design Concept
- the core part could be either a drug-containing unit fabricated by FDM or any formulation fed during fabrication (e.g., powders, pellets, gels);
- the soluble/erodible coat was based on a swellable/soluble hydrophilic polymer (i.e., HPC);
- the gastroresistant coat was based on an enteric-soluble polymer (i.e., EDR).

3.2. Set up of the Printing Process
3.2.1. Release-Controlling Coats
- temperature of the build plate and of the nozzle, which would determine the adhesion of the first layer to the build plate and that of successive layers between each other, thus impacting on the possibility of attaining a unitary item;
- number and height of deposited layers, which would impact on the dimensions (e.g., height, diameter, thickness of the coats) and resolution of the final system;
- flow rate, i.e., amount of material deposited over time, which would determine the sample weight;
- cooling rate, which is controlled by setting the speed of the cooling fan, and layer at which the selected rate was applied (activation layer), which overall would affect adhesion between successive layers;
- printing speed, which not only would directly determine the printing time but also may affect material adhesion.
3.2.2. Core
3.3. Chronotopic™ Prototypes for Pulsatile-Release and Colon-Targeting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Youan, B.-B.C. Chronopharmaceutics: Gimmick or clinically relevant approach to drug delivery? J. Control. Release 2004, 98, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Nainwal, N. Chronotherapeutics—A chronopharmaceutical approach to drug delivery in the treatment of asthma. J. Control. Release 2012, 163, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Sangalli, M.E.; Maroni, A.; Zema, L.; Cerea, M.; Gazzaniga, A. Chronotopic™ Technology. In Chronopharmaceutics: Science and Technology for Biological Rhythm-Guided Therapy and Prevention of Diseases; Youan, B.-B.C., Ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Maroni, A.; Zema, L.; Del Curto, M.D.; Loreti, G.; Gazzaniga, A. Oral pulsatile delivery: Rationale and chronopharmaceutical formulations. Int. J. Pharm. 2010, 398, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.S.; Biswas, N.; Karim, K.M.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Drug delivery system based on chronobiology—A review. J. Control. Release 2010, 147, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Ohdo, S.; Koyanagi, S.; Matsunaga, N.; Hamdan, A. Molecular basis of chronopharmaceutics. J. Pharm. Sci. 2011, 100, 3560–3576. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K. Small intestine transit time in the normal small bowel study. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1968, 104, 522–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, S.S.; Hardy, J.G.; Fara, J.W. Transit of pharmaceutical dosage forms through the small intestine. Gut 1986, 27, 886–892. [Google Scholar] [CrossRef] [Green Version]
- Sangalli, M.E.; Maroni, A.; Zema, L.; Busetti, C.; Giordano, F.; Gazzaniga, A. In vitro and in vivo evaluation of an oral system for time and/or site-specific drug delivery. J. Control. Release 2001, 73, 103–110. [Google Scholar] [CrossRef]
- Gazzaniga, A.; Maroni, A.; Sangalli, M.E.; Zema, L. Time-controlled oral delivery systems for colon targeting. Expert Opin. Drug Deliv. 2006, 3, 583–597. [Google Scholar] [CrossRef]
- Patel, M.M. Colon: A gateway for chronotherapeutic drug delivery systems. Expert Opin. Drug Deliv. 2015, 12, 1389–1395. [Google Scholar] [CrossRef] [Green Version]
- Del Curto, M.D.; Maroni, A.; Palugan, L.; Zema, L.; Gazzaniga, A.; Sangalli, M.E. Oral delivery system for two-pulse colonic release of protein drugs and protease inhibitor/absorption enhancer compounds. J. Pharm. Sci. 2011, 100, 3251–3259. [Google Scholar] [CrossRef]
- Maroni, A.; Zema, L.; Loreti, G.; Palugan, L.; Gazzaniga, A. Film coatings for oral pulsatile release. Int. J. Pharm. 2013, 457, 36–371. [Google Scholar] [CrossRef] [PubMed]
- Del Curto, M.D.; Palugan, L.; Foppoli, A.; Zema, L.; Gazzaniga, A.; Maroni, A. Erodible time-dependent colon delivery systems with improved efficiency in delaying the onset of drug release. J. Pharm. Sci. 2014, 103, 3585–3593. [Google Scholar] [CrossRef] [PubMed]
- Maroni, A.; Del Curto, M.D.; Salmaso, S.; Zema, L.; Melocchi, A.; Caliceti, P.; Gazzaniga, A. In vitro and in vivo evaluation of an oral multiple-unit formulation for colonic delivery of insulin. Eur. J. Pharm. Biopharm. 2016, 108, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Durán-Lobato, M.; Niu, Z.; Alonso, M.J. Oral Delivery of Biologics for Precision Medicine. Adv. Mater. 2020, 32, 1901935. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, A.; Sangalli, M.E.; Giordano, F. Oral Chronotopic® drug delivery systems: Achievement of time and/or site specificity. Eur. J. Pharm. Biopharm. 1994, 40, 246–250. [Google Scholar]
- Zema, L.; Maroni, A.; Foppoli, A.; Palugan, L.; Sangalli, M.E.; Gazzaniga, A. Different HPMC viscosity grades as coating agents for an oral time and/or site-controlled delivery system: An investigation into the mechanisms governing drug release. J. Pharm. Sci. 2007, 96, 1527–1536. [Google Scholar] [CrossRef]
- Foppoli, A.; Maroni, A.; Moutaharrik, S.; Melocchi, A.; Zema, L.; Palugan, L.; Cerea, M.; Gazzaniga, A. In vitro and human pharmacoscintigraphic evaluation of an oral 5-ASA delivery system for colonic release. Int. J. Pharm. 2019, 572, 118723. [Google Scholar] [CrossRef]
- Foppoli, A.; Maroni, A.; Palugan, L.; Zema, L.; Moutaharrik, S.; Melocchi, A.; Cerea, M.; Gazzaniga, A. Erodible coatings based on HPMC and cellulase for oral time-controlled release of drugs. Int. J. Pharm. 2020, 585, 119425. [Google Scholar] [CrossRef]
- Maroni, A.; Zema, L.; Cerea, M.; Foppoli, A.; Palugan, L.; Gazzaniga, A. Erodible drug delivery systems for time-controlled release into the gastrointestinal tract. J. Drug Deliv. Sci. Technol. 2016, 32, 229–235. [Google Scholar] [CrossRef]
- Foppoli, A.; Cerea, M.; Palugan, L.; Zema, L.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Evaluation of powder-layering vs. spray-coating techniques in the manufacturing of a swellable/erodible pulsatile delivery system. Drug Dev. Ind. Pharm. 2020, 46, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, A.; Cerea, M.; Cozzi, A.; Foppoli, A.; Maroni, A.; Zema, L. A novel injection-molded capsular device for oral pulsatile delivery based on swellable/erodible polymers. AAPS Pharm. Sci. Tech. 2011, 12, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zema, L.; Loreti, G.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Injection molding and its application to drug delivery. J. Control. Release 2012, 159, 324–331. [Google Scholar] [CrossRef]
- Zema, L.; Loreti, G.; Macchi, E.; Foppoli, A.; Maroni, A.; Gazzaniga, A. Injection-molded capsular device for oral pulsatile release: Development of a novel mold. J. Pharm. Sci. 2013, 102, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Prodduturi, S.; Manek, R.V.; Kolling, W.M.; Stodghill, S.P.; Repka, M.A. Water vapor sorption of hot-melt extruded hydroxypropyl cellulose films: Effect on physico-mechanical properties, release characteristics, and stability. J. Pharm. Sci. 2004, 93, 3047–3056. [Google Scholar] [CrossRef] [PubMed]
- Loreti, G.; Maroni, A.; Del Curto, M.D.; Melocchi, A.; Gazzaniga, A.; Zema, L. Evaluation of hot-melt extrusion technique in the preparation of HPC matrices for prolonged release. Eur. J. Pharm. Sci. 2014, 52, 77–85. [Google Scholar] [CrossRef]
- Macchi, E.; Zema, L.; Maroni, A.; Gazzaniga, A.; Felton, L.A. Enteric-coating of pulsatile-release HPC capsules prepared by injection molding. Eur. J. Pharm. Sci. 2015, 70, 1–11. [Google Scholar] [CrossRef]
- Macchi, E.; Zema, L.; Pandey, P.; Gazzaniga, A.; Felton, L.A. Influence of temperature and relative humidity conditions on the pan coating of hydroxypropyl cellulose molded capsules. Eur. J. Pharm. Biopharm. 2016, 100, 47–57. [Google Scholar] [CrossRef]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.W.; Ahmed, W.; Arafat, B. Emergence of 3D printed dosage forms: Opportunities and challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D printed medicines: A new branch of digital healthcare. Int. J. Pharm. 2018, 548, 586–596. [Google Scholar] [CrossRef]
- Zema, L.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Three-dimensional printing of medicinal products and the challenge of personalized therapy. J. Pharm. Sci. 2017, 106, 1697–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aho, J.; Bøtker, J.P.; Genina, N.; Edinger, M.; Arnfast, L.; Rantanen, J. Roadmap to 3D-printed oral pharmaceutical dosage forms: Feedstock filament properties and characterization for fused deposition modeling. J. Pharm. Sci. 2019, 108, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melocchi, A.; Uboldi, M.; Cerea, M.; Foppoli, A.; Maroni, A.; Moutaharrik, S.; Palugan, L.; Zema, L.; Gazzaniga, A. A Graphical review on the escalation of fused deposition modeling (fdm) 3d printing in the pharmaceutical field. J. Pharm. Sci. 2020, 109, 2943–2957. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Brambilla, D.; Leroux, J.-C. Is 3D printing of pharmaceuticals a disruptor or enabler? Adv. Mater. 2019, 31, e1805680. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing pharmaceuticals: Drug development to frontline care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Awad, A.; Madla, C.M.; Hatton, G.B.; Firth, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Shaping the future: Recent advances of 3D printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 2019, 16, 1081–1094. [Google Scholar] [CrossRef]
- Cailleaux, S.; Sanchez-Ballester, N.M.; Gueche, Y.A.; Bataille, B.; Soulairol, I. Fused Deposition Modeling (FDM), the new asset for the production of tailored medicines. J. Control. Release 2021, 330, 821–841. [Google Scholar] [CrossRef]
- Maroni, A.; Melocchi, A.; Zema, L.; Foppoli, A.; Gazzaniga, A. Retentive drug delivery systems based on shape memory materials. J. Appl. Polym. Sci. 2019, 137, 48798. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Inverardi, N.; Briatico-Vangosa, F.; Baldi, F.; Pandini, S.; Scalet, G.; Auricchio, F.; Cerea, M.; Foppoli, A.; et al. Expandable drug delivery system for gastric retention based on shape memory polymers: Development via 4D printing and extrusion. Int. J. Pharm. 2019, 571, 118700. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Maroni, A.; Foppoli, A.; Palugan, L.; Zema, L.; Gazzaniga, A. 3D printing by fused deposition modeling of single- and multi-compartment hollow systems for oral delivery—A review. Int. J. Pharm. 2020, 579, 119155. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Uboldi, M.; Cerea, M.; Foppoli, A.; Maroni, A.; Moutaharrik, S.; Palugan, L.; Zema, L.; Gazzaniga, A. Shape memory materials and 4D printing in pharmaceutics. Adv. Drug Deliv. Rev. 2021, 17, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Parietti, F.; Loreti, G.; Maroni, A.; Gazzaniga, A.; Zema, L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 360–367. [Google Scholar] [CrossRef]
- Maroni, A.; Melocchi, A.; Parietti, F.; Foppoli, A.; Zema, L.; Gazzaniga, A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J. Control. Release 2017, 268, 10–18. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Maccagnan, S.; Ortenzi, M.A.; Antenucci, S.; Briatico-Vangosa, F.; Maroni, A.; Gazzaniga, A.; Zema, L. Industrial Development of a 3D-printed nutraceutical delivery platform in the form of a multicompartment HPC capsule. AAPS Pharm. Sci. Tech. 2018, 19, 3343–3354. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Uboldi, M.; Parietti, F.; Cerea, M.; Foppoli, A.; Palugana, L.; Gazzaniga, A.; Maroni, A.; Zema, L. Lego-inspired capsular devices for the development of personalized dietary supplements: Proof of concept with multimodal release of caffeine. J. Pharm. Sci. 2020, 190, 1990–1999. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef]
- Feuerbach, T.; Kock, S.; Thommes, M. Characterisation of fused deposition modeling 3D printers for pharmaceutical and medical applications. Pharm. Dev. Technol. 2018, 23, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Briatico-Vangosa, F.; Uboldi, M.; Parietti, F.; Turchi, M.; von Zeppelin, D.; Maroni, A.; Zema, L.; Gazzaniga, A.; Zidan, A. Quality considerations on the pharmaceutical applications of fused deposition modeling 3D printing. Int. J. Pharm. 2021, 592, 119901. [Google Scholar] [CrossRef]
- Markl, D.; Zeitler, J.A.; Rasch, C.; Michaelsen, M.H.; Müllertz, A.; Rantanen, J.; Rades, T.; Bøtker, J. Analysis of 3D prints by x-ray computed microtomography and terahertz pulsed imaging. Pharm. Res. 2017, 34, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Markl, D.; Zeitler, A.; Rades, T.; Rantanen, J.; Bøtker, J. Toward quality assessment of 3D printed oral dosage forms. J. 3D Print. Med. 2018, 2, 27–33. [Google Scholar] [CrossRef]
- Smith, D.M.; Kapoor, Y.; Klinzing, G.R.; Procopio, A.T. Pharmaceutical 3D printing: Design and qualification of a single step print and fill capsule. Int. J. Pharm. 2018, 544, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Kapoor, Y.; Hermans, A.; Nofsinger, R.; Kesisoglou, F.; Gustafson, T.P.; Procopio, A. 3D printed capsules for quantitative regional absorption studies in the GI tract. Int. J. Pharm. 2018, 550, 418–428. [Google Scholar] [CrossRef]
- Melocchi, A.; Loreti, G.; Del Curto, M.D.; Maroni, A.; Gazzaniga, A.; Zema, L. Evaluation of hot-melt extrusion and injection molding for continuous manufacturing of immediate-release tablets. J. Pharm. Sci. 2015, 104, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Inverardi, N.; Uboldi, M.; Baldi, F.; Maroni, A.; Pandini, S.; Briatico-Vangosa, F.; Zema, L.; Gazzaniga, A. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly(vinyl alcohol): Design concept and 4D printing feasibility. Int. J. Pharm. 2019, 559, 299–311. [Google Scholar] [CrossRef]
- Zema, L.; Loreti, G.; Melocchi, A.; Maroni, A.; Palugan, L.; Gazzaniga, A. Gastroresistant capsular device prepared by injection molding. Int. J. Pharm. 2013, 440, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Casati, F.; Melocchi, A.; Moutaharrik, S.; Uboldi, M.; Foppoli, A.; Maroni, A.; Zema, L.; Neut, C.; Siepmann, F.; Siepmann, J.; et al. Injection molded capsules for colon delivery combining time-controlled and enzyme-triggered approaches. Int. J. Mol. Sci. 2020, 21, 1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misic, Z.; Muffler, K.; Sydow, G.; Kuentz, M. Novel starch-based PVA thermoplastic capsules for hydrophilic lipid-based formulations. J. Pharm. Sci. 2012, 101, 4516–4528. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Korte, C.; Quodbach, J. Formulation development and process analysis of drug-loaded filaments manufactured via hot-melt extrusion for 3D-printing of medicines. Pharm. Dev. Tech. 2018, 23, 1117–1127. [Google Scholar] [CrossRef]
- Korte, C.; Quodbach, J. 3D-printed network structures as controlled-release drug delivery systems: Dose adjustment, API release analysis and prediction. AAPS Pharm. Sci. Tech. 2018, 19, 3333–3342. [Google Scholar] [CrossRef] [PubMed]
- Novák, M.; Boleslavská, T.; Grof, Z.; Waněk, A.; Zadražil, A.; Beránek, J.; Kovačík, P.; Štěpánek, F. Virtual Prototyping and Parametric Design of 3D-Printed Tablets Based on the Solution of Inverse Problem. AAPS Pharm. Sci. Tech. 2018, 19, 3414–3424. [Google Scholar] [CrossRef] [PubMed]
- Negim, E.S.M.; Rakhmetullayeva, R.K.; Yeligbayeva GZh Urkimbaeva, P.I.; Primzharova, S.T.; Kaldybekov, D.B.; Khatib, J.M.; Mun, G.A.; Craig, W. Improving biodegradability of polyvinyl alcohol/starch blend films for packaging applications. Int. J. Sci. Basic Appl. Sci. 2014, 3, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Aldapa, C.A.; Velazquez, G.; Gutierrez, M.C.; Rangel-Vargas, E.; Castro-Rosas, J.; Aguirre-Loredo, R.Y. Effect of polyvinyl alcohol on the physicochemical properties of biodegradable starch films. Mater. Chem. Phys. 2020, 239, 122027. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S. Recent advances in starch, polyvinyl alcohol based polymer blends, nanocomposites and their biodegradability. Carbohydr. Polym. 2011, 85, 7–16. [Google Scholar] [CrossRef]
- Hilmi, F.F.; Wahit, M.U.; Shukri, N.A.; Ghazali, Z.; Zanuri, A.Z. Physico-chemical properties of biodegradable films of polyvinyl alcohol/sago starch for food packaging. Mater. Today 2019, 16, 1819–1824. [Google Scholar] [CrossRef]






| Formulation | T (°C) | Screw Speed (rpm) | Torque (N·cm) |
|---|---|---|---|
| HPC | 160 | 80 | 50 |
| EDR + 25% TEC | 165 | 80 | 120 |
| (PVA + 15% GLY) + 10% CFF | 185 | 70 | 110 |
| (PVA + 15% GLY) + 10% CFF + 30% EXP | 190 | 70 | 150 |
| (PVA + 15% GLY) + 10% CFF + 30% AMY | 190 | 70 | 140 |
| (HPC SSL + 5% PEG 400) + 10% CFF | 160 | 100 | 45 |
| (HPC SSL + 5% PEG 400) + 10% CFF + 30% EXP | 160 | 100 | 65 |
| Average | CV | Average Δ with Respect to the Electronic Model (%) | |||
|---|---|---|---|---|---|
| 600 µm—thick shells | Standard conditions | Weight (mg) | 128.12 | 9.23 | |
| External diameter (mm) | 10.38 | 3.89 | −7.32 | ||
| Height (mm) | 6.78 | 2.41 | +9.35 | ||
| Base thickness (µm) | 504 | 6 | −16.00 | ||
| Wall thickness (µm) | 448 | 8 | −25.33 | ||
| Fine-tuned conditions | Weight (mg) | 136.57 | 8.02 | ||
| External diameter (mm) | 11.33 | 1.82 | +1.16 | ||
| Height (mm) | 6.14 | 1.65 | +0.97 | ||
| Base thickness (µm) | 615 | 6 | +2.50 | ||
| Wall thickness (µm) | 572 | 7 | −4.67 | ||
| 900 µm—thick shells | Standard conditions | Weight (mg) | 201.13 | 12.55 | |
| External diameter (mm) | 10.85 | 3.87 | −3.13 | ||
| Height (mm) | 7.24 | 3.22 | +6.47 | ||
| Base thickness (µm) | 566 | 11 | −37.11 | ||
| Wall thickness (µm) | 1032 | 7 | +14.67 | ||
| Fine-tuned conditions | Weight (mg) | 187.81 | 7.14 | ||
| External diameter (mm) | 11.88 | 1.02 | +0.68 | ||
| Height (mm) | 6.91 | 1.35 | +1.62 | ||
| Base thickness (µm) | 897 | 4 | +0.33 | ||
| Wall thickness (µm) | 900 | 4 | +0.00 |
| Average | CV | Average Δ with Respect to the Electronic Model (%) | |||
|---|---|---|---|---|---|
| 400 µm—thick coatings | Standard conditions | Weight (mg) | 79.12 | 11.98 | |
| External diameter (mm) | 11.00 | 7.45 | −8.33 | ||
| Height (mm) | 7.45 | 2.98 | +6.43 | ||
| Base thickness (µm) | 563 | 14 | +40.75 | ||
| Wall thickness (µm) | 459 | 10 | +14.75 | ||
| Fine-tuned conditions | Weight (mg) | 76.0 | 7.0 | ||
| External diameter (mm) | 12.15 | 2.10 | −1.25 | ||
| Height (mm) | 7.13 | 1.64 | +1.85 | ||
| Base thickness (µm) | 398 | 6 | −2.25 | ||
| Wall thickness (µm) | 423 | 8 | +5.75 |
| Formulation | E, GPa (s.d.) | σ*, MPa (s.d.) |
|---|---|---|
| (PVA + 15% GLY) + 10% CFF | 0.41 (0.01) | 16.5 (0.70) |
| (PVA + 15% GLY) + 10% CFF + 30% EXP | 0.94 (0.09) | 13.8 (1.20) |
| (PVA + 15% GLY) + 10% CFF + 30% AMY | 1.29 (0.22) | 14.8 (5.30) |
| (HPC SSL + 5% PEG 400) + 10% CFF | 0.33 (0.08) | 3.6 (0.90) |
| (HPC SSL + 5% PEG 400) + 10% CFF + 30% EXP | 0.81 (0.17) | 3.2 (1.00) |
| Commercial PLA filament | 2.88 (0.09) | 43.4 (2.80) |
| Infill 100% | Infill 50% | |||
|---|---|---|---|---|
| Formulation | Eapp, GPa (s.d.) | σ*app, MPa (s.d.) | Eapp, GPa (s.d.) | σ*app, MPa (s.d.) |
| (PVA+15% GLY) + 10% CFF | 0.40 (0.05) | 15.5 (0.20) | 0.20 (0.10) | 5.5 (0.10) |
| (PVA+15% GLY) + 10% CFF + 30% AMY | 1.25 (0.03) | 20.7 (0.40) | 1.15 (0.06) | 13.2 (0.90) |
| (PVA+15% GLY) + 10% CFF + 30% EXP | 1.15 (0.06) a | 15.1 (0.90) | 0.64 (0.11) | 6.3 (0.50) |
| HPC SSL + 5% PEG 400) + 10% CFF | 0.53 (0.05) | 6.6 (0.90) | 0.22 (0.08) | 1.8 (0.18) |
| (HPC SSL + 5% PEG 400) + 10% CFF + 30% EXP | 0.29 (0.03) | 3.0 (0.60) | 0.06 (0.01) | 0.6 (0.20) |
| Formulation | (HPC SSL + 5% PEG 400) + 10% CFF | (PVA + 15% GLY) + 10% CFF | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| / | +30% EXP | / | +30% EXP | +30% AMY | |||||||||||
| Infill | 100% | 80% | 50% | 100% | 80% | 50% | 100% | 80% | 50% | 1005 | 80% | 50% | 100% | 80% | 50% |
| Weight, mg (CV) | 401.07 (6.61) | 389.84 (6.96) | 301.88 (7.84) | 464.81 (11.55) | 416.75 (10.42) | 351.78 (11.83) | 486.62 (2.92) | 433.73 (3.06) | 351.38 (8.019) | 434.95 (2.34) | 467.06 (5.95) | 326.89 (6.89) | 418.51 (1.01) | 486.90 (4.12) | 302.52 (5.11) |
| Height, mm (CV) | 5.68 (0.99) | 5.20 (1.90) | 5.55 (1.99) | 5.28 (2.33) | 5.61 (3.97) | 5.21 (4.93) | 5.67 (1.73) | 4.99 (2.18) | 5.15 (3.96) | 5.33 (2.35) | 5.17 (3.38) | 5.06 (2.24) | 5.32 (1.68) | 5.45 (2.20) | 5.14 (6.55) |
| Diameter, mm (CV) | 9.89 (1.40) | 9.98 (1.74) | 9.99 (3.51) | 10.06 (1.30) | 10.18 (1.43) | 10.08 (1.88) | 10.31 (1.28) | 10.32 (1.30)) | 10.28 (1.90) | 9.83 (0.78) | 9.43 (1.55) | 9.84 (2.02) | 9.88 (1.17) | 10.00 (1.06) | 9.93 (3.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melocchi, A.; Uboldi, M.; Briatico-Vangosa, F.; Moutaharrik, S.; Cerea, M.; Foppoli, A.; Maroni, A.; Palugan, L.; Zema, L.; Gazzaniga, A. The Chronotopic™ System for Pulsatile and Colonic Delivery of Active Molecules in the Era of Precision Medicine: Feasibility by 3D Printing via Fused Deposition Modeling (FDM). Pharmaceutics 2021, 13, 759. https://doi.org/10.3390/pharmaceutics13050759
Melocchi A, Uboldi M, Briatico-Vangosa F, Moutaharrik S, Cerea M, Foppoli A, Maroni A, Palugan L, Zema L, Gazzaniga A. The Chronotopic™ System for Pulsatile and Colonic Delivery of Active Molecules in the Era of Precision Medicine: Feasibility by 3D Printing via Fused Deposition Modeling (FDM). Pharmaceutics. 2021; 13(5):759. https://doi.org/10.3390/pharmaceutics13050759
Chicago/Turabian StyleMelocchi, Alice, Marco Uboldi, Francesco Briatico-Vangosa, Saliha Moutaharrik, Matteo Cerea, Anastasia Foppoli, Alessandra Maroni, Luca Palugan, Lucia Zema, and Andrea Gazzaniga. 2021. "The Chronotopic™ System for Pulsatile and Colonic Delivery of Active Molecules in the Era of Precision Medicine: Feasibility by 3D Printing via Fused Deposition Modeling (FDM)" Pharmaceutics 13, no. 5: 759. https://doi.org/10.3390/pharmaceutics13050759
APA StyleMelocchi, A., Uboldi, M., Briatico-Vangosa, F., Moutaharrik, S., Cerea, M., Foppoli, A., Maroni, A., Palugan, L., Zema, L., & Gazzaniga, A. (2021). The Chronotopic™ System for Pulsatile and Colonic Delivery of Active Molecules in the Era of Precision Medicine: Feasibility by 3D Printing via Fused Deposition Modeling (FDM). Pharmaceutics, 13(5), 759. https://doi.org/10.3390/pharmaceutics13050759