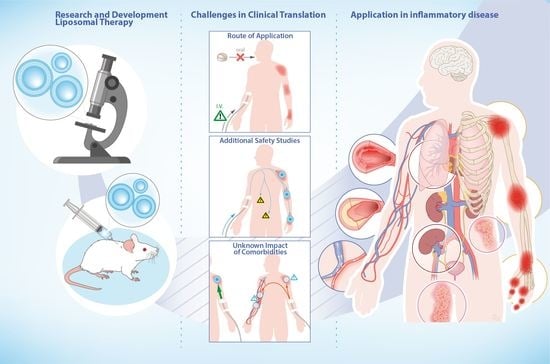
Recent Advances in Liposomal-Based Anti-Inflammatory Therapy
Abstract
:1. Introduction
2. Innovations in Liposome Formulation
2.1. The Liposome Lipid Bilayer
- (a)
- (b)
- (c)
- (d)
- (e)
2.2. Liposome Manufacturing and Quality Control
3. Application in Inflammatory Disease
3.1. Rheumatoid Arthritis
3.2. Psoriasis
3.3. Vascular Inflammation
3.4. Solid Organ Transplantation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Germain, M.; Caputo, F.; Metcalfe, S.; Tosi, G.; Spring, K.; Åslund, A.K.O.; Pottier, A.; Schiffelers, R.; Ceccaldi, A.; Schmid, R. Delivering the Power of Nanomedicine to Patients Today. J. Control. Release 2020, 326, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, N.; Li, Q.; Zhou, Y.; Luan, Y. A Two-Pronged Photodynamic Nanodrug to Prevent Metastasis of Basal-like Breast Cancer. Chem. Commun. 2021, 57, 2305–2308. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, N.; Zhou, Y.; Song, A.; Jin, G.; Li, Z.; Luan, Y. An Injectable Hydrogel Using an Immunomodulating Gelator for Amplified Tumor Immunotherapy by Blocking the Arginase Pathway. Acta Biomater. 2021, 124, 179–190. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, K.; Kanazawa, K. AmBisome: Relationship between the Pharmacokinetic Characteristics Acquired by Liposomal Formulation and Safety/Efficacy. J. Liposome Res. 2017, 27, 186–194. [Google Scholar] [CrossRef]
- Hartrick, C.T.; Hartrick, K.A. Extended-Release Epidural Morphine (DepoDurTM): Review and Safety Analysis. Expert Rev. Neurother. 2008, 8, 1641–1648. [Google Scholar] [CrossRef]
- Schirrmacher, V. From Chemotherapy to Biological Therapy: A Review of Novel Concepts to Reduce the Side Effects of Systemic Cancer Treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side Effects and Toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Hu, X.; Saeed, M.; Chen, B.; Li, Y.; Yu, H. Overview of Recent Advances in Liposomal Nanoparticle-Based Cancer Immunotherapy. Acta Pharmacol. Sin. 2019, 40, 1129–1137. [Google Scholar] [CrossRef] [Green Version]
- Vijaykumar, N.; Sandeep, K. Recent Advances in Liposomal Drug Delivery: A Review. Pharm. Nanotechnol. 2015, 3, 35–55. [Google Scholar]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Everts, M.; Koning, G.A.; Kok, R.J.; Asgeirsdóttir, S.A.; Vestweber, D.; Meijer, D.K.F.; Storm, G.; Molema, G. In Vitro Cellular Handling and in Vivo Targeting of E-Selectin-Directed Immunoconjugates and Immunoliposomes Used for Drug Delivery to Inflamed Endothelium. Pharm. Res. 2003, 20, 64–72. [Google Scholar] [CrossRef]
- Lum, H.; Malik, A.B. Regulation of Vascular Endothelial Barrier Function. Am. J. Physiol. 1994, 267, L223–L241. [Google Scholar] [CrossRef]
- Antohe, F.; Lin, L.; Kao, G.Y.; Poznansky, M.J.; Allen, T.M. Transendothelial Movement of Liposomes In Vitro Mediated by Cancer Cells, Neutrophils or Histamine. J. Liposome Res. 2004, 14, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and Challenges towards Targeted Delivery of Cancer Therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.-T.; Gao, F.; Gu, K.; Chen, D.-K. The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Tackling the Various Classes of Nano-Therapeutics Employed in Topical Therapy of Psoriasis. Drug Deliv. 2020, 27, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A.S.; Ratna, B.R.; Kahn, B. Self-Assembling Phospholipid Filaments. Nature 1991, 352, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Gregoriadis, G. Engineering Liposomes for Drug Delivery: Progress and Problems. Trends Biotechnol. 1995, 13, 527–537. [Google Scholar] [CrossRef]
- Jain, A.; Hurkat, P.; Jain, S.K. Development of Liposomes Using Formulation by Design: Basics to Recent Advances. Chem. Phys. Lipids 2019, 224, 104764. [Google Scholar] [CrossRef] [PubMed]
- Juszkiewicz, K.; Sikorski, A.F.; Czogalla, A. Building Blocks to Design Liposomal Delivery Systems. Int. J. Mol. Sci. 2020, 21, 9559. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Hansen, C.; Martin, F.; Redemann, C.; Yau-Young, A. Liposomes Containing Synthetic Lipid Derivatives of Poly(Ethylene Glycol) Show Prolonged Circulation Half-Lives in vivo. Biochim. Biophys. Acta BBA Biomembr. 1991, 1066, 29–36. [Google Scholar] [CrossRef]
- Allen, T.M.; Hansen, C. Pharmacokinetics of Stealth versus Conventional Liposomes: Effect of Dose. Biochim. Biophys. Acta 1991, 1068, 133–141. [Google Scholar] [CrossRef]
- Europe PMC. A New Strategy for Attachment of Antibodies to Sterically Stabilized Liposomes Resulting in Efficient Targeting to Cancer Cells—Abstract. Available online: https://europepmc.org/article/MED/7632714 (accessed on 5 June 2021).
- Di, J.; Xie, F.; Xu, Y. When Liposomes Met Antibodies: Drug Delivery and Beyond. Adv. Drug Deliv. Rev. 2020, 154–155, 151–162. [Google Scholar] [CrossRef]
- Ewert, K.K.; Zidovska, A.; Ahmad, A.; Bouxsein, N.F.; Evans, H.M.; McAllister, C.S.; Samuel, C.E.; Safinya, C.R. Cationic Liposome-Nucleic Acid Complexes for Gene Delivery and Silencing: Pathways and Mechanisms for Plasmid DNA and SiRNA. Top. Curr. Chem. 2010, 296, 191–226. [Google Scholar] [CrossRef] [PubMed]
- Safinya, C.R.; Ewert, K.K.; Majzoub, R.N.; Leal, C. Cationic Liposome–Nucleic Acid Complexes for Gene Delivery and Gene Silencing. New J. Chem. 2014, 38, 5164–5172. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, M.M.A.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Deformable Liposomes and Ethosomes: Mechanism of Enhanced Skin Delivery. Int. J. Pharm. 2006, 322, 60–66. [Google Scholar] [CrossRef]
- Ashtikar, M.; Nagarsekar, K.; Fahr, A. Transdermal Delivery from Liposomal Formulations—Evolution of the Technology over the Last Three Decades. J. Control. Release 2016, 242, 126–140. [Google Scholar] [CrossRef]
- Lee, Y.; Thompson, D.H. Stimuli-Responsive Liposomes for Drug Delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef]
- Antoniou, A.I.; Giofrè, S.; Seneci, P.; Passarella, D.; Pellegrino, S. Stimulus-Responsive Liposomes for Biomedical Applications. Drug Discov. Today 2021. [Google Scholar] [CrossRef]
- Blom, A.B.; van Lent, P.L.E.M.; Holthuysen, A.E.M.; van der Kraan, P.M.; Roth, J.; van Rooijen, N.; van den Berg, W.B. Synovial Lining Macrophages Mediate Osteophyte Formation during Experimental Osteoarthritis. Osteoarthr. Cartil. 2004, 12, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Barrera, P.; Blom, A.; van Lent, P.L.; van Bloois, L.; Beijnen, J.H.; van Rooijen, N.; de Waal Malefijt, M.C.; van de Putte, L.B.; Storm, G.; van den Berg, W.B. Synovial Macrophage Depletion with Clodronate-Containing Liposomes in Rheumatoid Arthritis. Arthritis Rheum. 2000, 43, 1951–1959. [Google Scholar] [CrossRef] [Green Version]
- Van den Hoven, J.M.; Van Tomme, S.R.; Metselaar, J.M.; Nuijen, B.; Beijnen, J.H.; Storm, G. Liposomal Drug Formulations in the Treatment of Rheumatoid Arthritis. Mol. Pharm. 2011, 8, 1002–1015. [Google Scholar] [CrossRef]
- Ozbakir, B.; Crielaard, B.J.; Metselaar, J.M.; Storm, G.; Lammers, T. Liposomal Corticosteroids for the Treatment of Inflammatory Disorders and Cancer. J. Control. Release 2014, 190, 624–636. [Google Scholar] [CrossRef]
- Presumey, J.; Duroux-Richard, I.; Courties, G.; Apparailly, F. Cationic Liposome Formulations for RNAi-Based Validation of Therapeutic Targets in Rheumatoid Arthritis. Curr. Opin. Mol. Ther. 2010, 12, 325–330. [Google Scholar] [PubMed]
- Liu, H.; Kang, R.S.; Bagnowski, K.; Yu, J.M.; Radecki, S.; Daniel, W.L.; Anderson, B.R.; Nallagatla, S.; Schook, A.; Agarwal, R.; et al. Targeting the IL-17 Receptor Using Liposomal Spherical Nucleic Acids as Topical Therapy for Psoriasis. J. Investig. Dermatol. 2020, 140, 435–444.e4. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Santos, A.C.; Silva, A.L.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Castro, R.; Veiga, F. Ethosomes as Nanocarriers for the Development of Skin Delivery Formulations. Pharm. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nayak, N.; Somanna, P.; Patil, A.B.; Radhakrishnan, A. Progress in Novel Ultradeformable Vesicular Drug Carrier in the Topical and Transdermal Treatment of Psoriasis. Ther. Deliv 2020, 11, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, K.; Kato, Y.; Onishi, H.; Machida, Y. In Vitro Characteristics of Liposomes and Double Liposomes Prepared Using a Novel Glass Beads Method. J. Control. Release 2003, 90, 71–79. [Google Scholar] [CrossRef]
- Gentine, P.; Bubel, A.; Crucifix, C.; Bourel-Bonnet, L.; Frisch, B. Manufacture of Liposomes by Isopropanol Injection: Characterization of the Method. J. Liposome Res. 2012, 22, 18–30. [Google Scholar] [CrossRef]
- Wagner, A.; Platzgummer, M.; Kreismayr, G.; Quendler, H.; Stiegler, G.; Ferko, B.; Vecera, G.; Vorauer-Uhl, K.; Katinger, H. GMP Production of Liposomes—A New Industrial Approach. J. Liposome Res. 2006, 16, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Kastner, E.; Verma, V.; Lowry, D.; Perrie, Y. Microfluidic-Controlled Manufacture of Liposomes for the Solubilisation of a Poorly Water Soluble Drug. Int. J. Pharm. 2015, 485, 122–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zizzari, A.; Bianco, M.; Carbone, L.; Perrone, E.; Amato, F.; Maruccio, G.; Rendina, F.; Arima, V. Continuous-Flow Production of Injectable Liposomes via a Microfluidic Approach. Materials 2017, 10, 1411. [Google Scholar] [CrossRef] [Green Version]
- Yanar, F.; Mosayyebi, A.; Nastruzzi, C.; Carugo, D.; Zhang, X. Continuous-Flow Production of Liposomes with a Millireactor under Varying Fluidic Conditions. Pharmaceutics 2020, 12, 1001. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent Advances on Liposomal Nanoparticles: Synthesis, Characterization and Biomedical Applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samad, A.; Sultana, Y.; Aqil, M. Liposomal Drug Delivery Systems: An Update Review. Curr. Drug Deliv. 2007, 4, 297–305. [Google Scholar] [CrossRef]
- Rozo, A.J.; Cox, M.H.; Devitt, A.; Rothnie, A.J.; Goddard, A.D. Biophysical Analysis of Lipidic Nanoparticles. Methods 2020, 180, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Fehér, B.; Kitka, D.; Wacha, A.; Bóta, A.; Berényi, S.; Pipich, V.; Fraikin, J.-L. Size Measurement of Extracellular Vesicles and Synthetic Liposomes: The Impact of the Hydration Shell and the Protein Corona. Colloids Surf. B Biointerfaces 2020, 192, 111053. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An Overview of Liposome Lyophilization and Its Future Potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef]
- Ulrich, A.S. Biophysical Aspects of Using Liposomes as Delivery Vehicles. Biosci. Rep. 2002, 22, 129–150. [Google Scholar] [CrossRef]
- Wilhelm, D.L. Mechanisms Responsible for Increased Vascular Permeability in Acute Inflammation. Agents Actions 1973, 3, 297–306. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Inflammation and the Blood Microvascular System. Cold Spring Harb Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in Inflammation, Repair and Regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Van Alem, C.M.A.; Boonstra, M.; Prins, J.; Bezhaeva, T.; van Essen, M.F.; Ruben, J.M.; Vahrmeijer, A.L.; van der Veer, E.P.; de Fijter, J.W.; Reinders, M.E.; et al. Local Delivery of Liposomal Prednisolone Leads to an Anti-Inflammatory Profile in Renal Ischaemia-Reperfusion Injury in the Rat. Nephrol. Dial. Transplant. 2018, 33, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2019, 27, 501–507. [Google Scholar] [CrossRef] [PubMed]
- WHO. Chronic Rheumatic Conditions. Available online: http://www.who.int/chp/topics/rheumatic/en/ (accessed on 19 March 2021).
- Thakur, S.; Riyaz, B.; Patil, A.; Kaur, A.; Kapoor, B.; Mishra, V. Novel Drug Delivery Systems for NSAIDs in Management of Rheumatoid Arthritis: An Overview. Biomed. Pharmacother. 2018, 106, 1011–1023. [Google Scholar] [CrossRef]
- Kapoor, B.; Singh, S.K.; Gulati, M.; Gupta, R.; Vaidya, Y. Application of Liposomes in Treatment of Rheumatoid Arthritis: Quo Vadis. Sci. World J. 2014, 2014, e978351. [Google Scholar] [CrossRef] [Green Version]
- Scheiman, J.M.; Hindley, C.E. Strategies to Optimize Treatment with NSAIDs in Patients at Risk for Gastrointestinal and Cardiovascular Adverse Events. Clin. Ther. 2010, 32, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.; Mohamed, A.A.A.; Emery, P. Glucocorticoids and Rheumatoid Arthritis. Rheum. Dis. Clin. 2016, 42, 33–46. [Google Scholar] [CrossRef]
- Saag, K.G.; Criswell, L.A.; Sems, K.M.; Nettleman, M.D.; Kolluri, S. Low-Dose Corticosteroids in Rheumatoid Arthritis. A Meta-Analysis of Their Moderate-Term Effectiveness. Arthritis Rheum. 1996, 39, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Caplan, L.; Wolfe, F.; Russell, A.S.; Michaud, K. Corticosteroid Use in Rheumatoid Arthritis: Prevalence, Predictors, Correlates, and Outcomes. J. Rheumatol. 2007, 34, 696–705. [Google Scholar]
- Ethgen, O.; de Esteves, F.L.; Bruyere, O.; Reginster, J.-Y. What Do We Know about the Safety of Corticosteroids in Rheumatoid Arthritis? Curr. Med Res. Opin. 2013, 29, 1147–1160. [Google Scholar] [CrossRef]
- van den Hoven, J.M.; Hofkens, W.; Wauben, M.H.M.; Wagenaar-Hilbers, J.P.A.; Beijnen, J.H.; Nuijen, B.; Metselaar, J.M.; Storm, G. Optimizing the Therapeutic Index of Liposomal Glucocorticoids in Experimental Arthritis. Int. J. Pharm. 2011, 416, 471–477. [Google Scholar] [CrossRef]
- Koning, G.A.; Schiffelers, R.M.; Wauben, M.H.M.; Kok, R.J.; Mastrobattista, E.; Molema, G.; ten Hagen, T.L.M.; Storm, G. Targeting of Angiogenic Endothelial Cells at Sites of Inflammation by Dexamethasone Phosphate-Containing RGD Peptide Liposomes Inhibits Experimental Arthritis. Arthritis Rheum 2006, 54, 1198–1208. [Google Scholar] [CrossRef] [Green Version]
- Metselaar, J.M.; van den Berg, W.B.; Holthuysen, A.E.M.; Wauben, M.H.M.; Storm, G.; van Lent, P.L.E.M. Liposomal Targeting of Glucocorticoids to Synovial Lining Cells Strongly Increases Therapeutic Benefit in Collagen Type II Arthritis. Ann. Rheum. Dis. 2004, 63, 348–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, O.; Bansal, P.; Goyal, A.; Lappin, S.L. Disease Modifying Anti-Rheumatic Drugs (DMARD). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Plosker, G.L.; Croom, K.F. Sulfasalazine: A Review of Its Use in the Management of Rheumatoid Arthritis. Drugs 2005, 65, 1825–1849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yuan, R.; Li, C.; Wei, W.; Shen, W.; Cui, Y.; Yuan, X. Macrophage Depletion with Clodronate-Containing Liposomes Affects the Incidence and Development of Rheumatoid Arthritis. Z. Rheumatol. 2019, 78, 996–1003. [Google Scholar] [CrossRef]
- Sung, J.Y.; Hong, J.H.; Kang, H.S.; Choi, I.; Lim, S.D.; Lee, J.K.; Seok, J.H.; Lee, J.H.; Hur, G.M. Methotrexate Suppresses the Interleukin-6 Induced Generation of Reactive Oxygen Species in the Synoviocytes of Rheumatoid Arthritis. Immunopharmacology 2000, 47, 35–44. [Google Scholar] [CrossRef]
- Lee, E.B.; Fleischmann, R.; Hall, S.; Wilkinson, B.; Bradley, J.D.; Gruben, D.; Koncz, T.; Krishnaswami, S.; Wallenstein, G.V.; Zang, C.; et al. Tofacitinib versus Methotrexate in Rheumatoid Arthritis. N. Engl. J. Med. 2014, 370, 2377–2386. [Google Scholar] [CrossRef] [Green Version]
- Lane, J.C.E.; Weaver, J.; Kostka, K.; Duarte-Salles, T.; Abrahao, M.T.F.; Alghoul, H.; Alser, O.; Alshammari, T.M.; Biedermann, P.; Banda, J.M.; et al. Risk of Hydroxychloroquine Alone and in Combination with Azithromycin in the Treatment of Rheumatoid Arthritis: A Multinational, Retrospective Study. Lancet Rheumatol. 2020, 2, e698–e711. [Google Scholar] [CrossRef]
- Halloran, P.F. Molecular Mechanisms of New Immunosuppressants. Clin. Transplant. 1996, 10, 118–123. [Google Scholar]
- Maddison, P.; Kiely, P.; Kirkham, B.; Lawson, T.; Moots, R.; Proudfoot, D.; Reece, R.; Scott, D.; Sword, R.; Taggart, A.; et al. Leflunomide in Rheumatoid Arthritis: Recommendations through a Process of Consensus. Rheumatology 2005, 44, 280–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.; Goodfellow, R.; Topley, N.; Amos, N.; Williams, B. The Suppression of Rat Collagen-Induced Arthritis and Inhibition of Macrophage Derived Mediator Release by Liposomal Methotrexate Formulations. Inflamm. Res. 2000, 49, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, P.; Shetty, R.; Koland, M.; Vijayanarayana, K.; Vijayalakshmi, K.; Nairy, M.H.; Nisha, G. Investigation of Nano Lipid Vesicles of Methotrexate for Anti-Rheumatoid Activity. Int. J. Nanomed. 2012, 7, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deviatkin, A.A.; Vakulenko, Y.A.; Akhmadishina, L.V.; Tarasov, V.V.; Beloukhova, M.I.; Zamyatnin Jr., A. A.; Lukashev, A.N. Emerging Concepts and Challenges in Rheumatoid Arthritis Gene Therapy. Biomedicines 2020, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barba, A.A.; Bochicchio, S.; Dalmoro, A.; Lamberti, G. Lipid Delivery Systems for Nucleic-Acid-Based-Drugs: From Production to Clinical Applications. Pharmaceutics 2019, 11, 360. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Silva, M.; Faria-Silva, C.; Viana Baptista, P.; Fernandes, E.; Ramos Fernandes, A.; Corvo, M.L. Liposomal Nanosystems in Rheumatoid Arthritis. Pharmaceutics 2021, 13, 454. [Google Scholar] [CrossRef]
- Rahman, M.; Kumar, V.; Beg, S.; Sharma, G.; Katare, O.P.; Anwar, F. Emergence of Liposome as Targeted Magic Bullet for Inflammatory Disorders: Current State of the Art. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1597–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.; Beg, S.; Sharma, G.; Anwar, F.; Kumar, V. Emergence of Lipid-Based Vesicular Carriers as Nanoscale Pharmacotherapy in Rheumatoid Arthritis. Recent Pat. Nanomed. 2015, 5. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, X.; Qi, J.; Shu, G.; Du, Y.; Ying, X. Sinomenine Hydrochloride Loaded Thermosensitive Liposomes Combined with Microwave Hyperthermia for the Treatment of Rheumatoid Arthritis. Int. J. Pharm. 2020, 576, 119001. [Google Scholar] [CrossRef]
- Gouveia, V.M.; Lopes-de-Araújo, J.; Costa Lima, S.A.; Nunes, C.; Reis, S. Hyaluronic Acid-Conjugated PH-Sensitive Liposomes for Targeted Delivery of Prednisolone on Rheumatoid Arthritis Therapy. Nanomedicine 2018, 13, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; He, Y.; Wu, H.; Zhou, M.; Xu, Z.; Xiong, R.; Yan, F.; Liu, H. Near-Infrared Fluorescence Imaging-Guided Focused Ultrasound-Mediated Therapy against Rheumatoid Arthritis by MTX-ICG-Loaded IRGD-Modified Echogenic Liposomes. Theranostics 2020, 10, 10092–10105. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Amerigos, J.C.K.D.; Su, Z.; Guissi, N.E.I.; Xiao, Y.; Zong, L.; Ping, Q. Folate Receptor-Targeting and Reactive Oxygen Species-Responsive Liposomal Formulation of Methotrexate for Treatment of Rheumatoid Arthritis. Pharmaceutics 2019, 11, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Jain, A.; Tiwari, A.; Saraf, S.; Panda, P.K.; Agrawal, G.P.; Jain, S.K. Folate Conjugated Double Liposomes Bearing Prednisolone and Methotrexate for Targeting Rheumatoid Arthritis. Pharm. Res. 2019, 36, 123. [Google Scholar] [CrossRef]
- Duan, W.; Li, H. Combination of NF-KB Targeted SiRNA and Methotrexate in a Hybrid Nanocarrier towards the Effective Treatment in Rheumatoid Arthritis. J. Nanobiotechnol. 2018, 16, 58. [Google Scholar] [CrossRef]
- Zhang, Y.; He, W.; Du, Y.; Du, Y.; Zhao, C.; Zhang, Y.; Zhang, H.; Yin, L.; Li, X. Dimeric Artesunate Phospholipid-Conjugated Liposomes as Promising Anti-Inflammatory Therapy for Rheumatoid Arthritis. Int. J. Pharm. 2020, 579, 119178. [Google Scholar] [CrossRef]
- Mohanty, S.; Sahoo, A.K.; Konkimalla, V.B.; Pal, A.; Si, S.C. Correction to Naringin in Combination with Isothiocyanates as Liposomal Formulations Potentiates the Anti-Inflammatory Activity in Different Acute and Chronic Animal Models of Rheumatoid Arthritis. ACS Omega 2021, 6, 3434. [Google Scholar] [CrossRef]
- Kapoor, B.; Gulati, M.; Singh, S.K.; Khatik, G.L.; Gupta, R.; Kumar, R.; Kumar, R.; Gowthamarajan, K.; Mahajan, S.; Gupta, S. Fail-Safe Nano-Formulation of Prodrug of Sulfapyridine: Preparation and Evaluation for Treatment of Rheumatoid Arthritis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111332. [Google Scholar] [CrossRef]
- Hofkens, W.; Grevers, L.C.; Walgreen, B.; de Vries, T.J.; Leenen, P.J.M.; Everts, V.; Storm, G.; van den Berg, W.B.; van Lent, P.L. Intravenously Delivered Glucocorticoid Liposomes Inhibit Osteoclast Activity and Bone Erosion in Murine Antigen-Induced Arthritis. J. Control. Release 2011, 152, 363–369. [Google Scholar] [CrossRef]
- Rauchhaus, U.; Schwaiger, F.W.; Panzner, S. Separating Therapeutic Efficacy from Glucocorticoid Side-Effects in Rodent Arthritis Using Novel, Liposomal Delivery of Dexamethasone Phosphate: Long-Term Suppression of Arthritis Facilitates Interval Treatment. Arthritis Res. Ther. 2009, 11, R190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avnir, Y.; Ulmansky, R.; Wasserman, V.; Even-Chen, S.; Broyer, M.; Barenholz, Y.; Naparstek, Y. Amphipathic Weak Acid Glucocorticoid Prodrugs Remote-Loaded into Sterically Stabilized Nanoliposomes Evaluated in Arthritic Rats and in a Beagle Dog: A Novel Approach to Treating Autoimmune Arthritis. Arthritis Rheum. 2008, 58, 119–129. [Google Scholar] [CrossRef]
- Komano, Y.; Yagi, N.; Onoue, I.; Kaneko, K.; Miyasaka, N.; Nanki, T. Arthritic Joint-Targeting Small Interfering RNA-Encapsulated Liposome: Implication for Treatment Strategy for Rheumatoid Arthritis. J. Pharmacol. Exp. Ther. 2012, 340, 109–113. [Google Scholar] [CrossRef]
- Khoury, M.; Escriou, V.; Courties, G.; Galy, A.; Yao, R.; Largeau, C.; Scherman, D.; Jorgensen, C.; Apparailly, F. Efficient Suppression of Murine Arthritis by Combined Anticytokine Small Interfering RNA Lipoplexes. Arthritis Rheum. 2008, 58, 2356–2367. [Google Scholar] [CrossRef]
- Khoury, M.; Louis-Plence, P.; Escriou, V.; Noel, D.; Largeau, C.; Cantos, C.; Scherman, D.; Jorgensen, C.; Apparailly, F. Efficient New Cationic Liposome Formulation for Systemic Delivery of Small Interfering RNA Silencing Tumor Necrosis Factor Alpha in Experimental Arthritis. Arthritis Rheum. 2006, 54, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, B.; Huang, J.; Xiang, X.; Tang, Y.; Ma, L.; Yan, F.; Cheng, C.; Qiu, L. Ultrasound-Targeted Microbubble Destruction Augmented Synergistic Therapy of Rheumatoid Arthritis via Targeted Liposomes. J. Mater. Chem. B 2020, 8, 5245–5256. [Google Scholar] [CrossRef] [PubMed]
- Van der Geest, T.; Metselaar, J.M.; Gerrits, D.; van Lent, P.L.; Storm, G.; Laverman, P.; Boerman, O.C. [18]F FDG PET/CT Imaging to Monitor the Therapeutic Effect of Liposome-Encapsulated Prednisolone in Experimental Rheumatoid Arthritis. J. Control. Release 2015, 209, 20–26. [Google Scholar] [CrossRef]
- Wang, S.; Yang, S.; Lai, X.; Song, Y.; Hu, L.; Li, C.; Shi, T.; Liu, X.; Deng, Y.; Chen, G. Sialic Acid Conjugate–Modified Liposomal Dexamethasone Palmitate Targeting Neutrophils for Rheumatoid Arthritis Therapy: Influence of Particle Size. AAPS PharmSciTech 2021, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Luo, X.; Zhou, S.; Zhu, J.; Xiao, M.; Li, C.; Zheng, H.; Qiu, Q.; Lai, C.; Liu, X.; et al. Neutrophil-Mediated Delivery of Dexamethasone Palmitate-Loaded Liposomes Decorated with a Sialic Acid Conjugate for Rheumatoid Arthritis Treatment. Pharm. Res. 2019, 36, 97. [Google Scholar] [CrossRef]
- Meka, R.R.; Venkatesha, S.H.; Acharya, B.; Moudgil, K.D. Peptide-Targeted Liposomal Delivery of Dexamethasone for Arthritis Therapy. Nanomedicine 2019, 14, 1455–1469. [Google Scholar] [CrossRef] [PubMed]
- Poh, S.; Chelvam, V.; Kelderhouse, L.E.; Ayala-López, W.; Vaitilingam, B.; Putt, K.S.; Low, P.S. Folate-Conjugated Liposomes Target and Deliver Therapeutics to Immune Cells in a Rat Model of Rheumatoid Arthritis. Nanomedicine 2017, 12, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Vanniasinghe, A.S.; Manolios, N.; Schibeci, S.; Lakhiani, C.; Kamali-Sarvestani, E.; Sharma, R.; Kumar, V.; Moghaddam, M.; Ali, M.; Bender, V. Targeting Fibroblast-like Synovial Cells at Sites of Inflammation with Peptide Targeted Liposomes Results in Inhibition of Experimental Arthritis. Clin. Immunol. 2014, 151, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, E.; Lager, F.; Le Roux, D.; Nogueira, P.; Freitas, J.; Charvet, C.; Renault, G.; Loureiro, A.; Almeida, C.R.; Ohradanova-Repic, A.; et al. Enhancing Methotrexate Tolerance with Folate Tagged Liposomes in Arthritic Mice. J. Biomed. Nanotechnol. 2015, 11, 2243–2252. [Google Scholar] [CrossRef] [Green Version]
- Xue, L.; Wang, D.; Zhang, X.; Xu, S.; Zhang, N. Targeted and Triple Therapy-Based Liposomes for Enhanced Treatment of Rheumatoid Arthritis. Int. J. Pharm. 2020, 586, 119642. [Google Scholar] [CrossRef] [PubMed]
- Meka, R.R.; Venkatesha, S.H.; Moudgil, K.D. Peptide-Directed Liposomal Delivery Improves the Therapeutic Index of an Immunomodulatory Cytokine in Controlling Autoimmune Arthritis. J. Control. Release 2018, 286, 279–288. [Google Scholar] [CrossRef]
- Martinez-Lostao, L.; García-Alvarez, F.; Basáñez, G.; Alegre-Aguarón, E.; Desportes, P.; Larrad, L.; Naval, J.; Martínez-Lorenzo, M.J.; Anel, A. Liposome-Bound APO2L/TRAIL Is an Effective Treatment in a Rabbit Model of Rheumatoid Arthritis. Arthritis Rheum. 2010, 62, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Christophers, E. Psoriasis—Epidemiology and Clinical Spectrum. Clin. Exp. Dermatol. 2001, 26, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Furue, K.; Ito, T.; Tsuji, G.; Kadono, T.; Nakahara, T.; Furue, M. Autoimmunity and Autoimmune Co-morbidities in Psoriasis. Immunology 2018, 154, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Haustein, U.F.; Rytter, M. Methotrexate in Psoriasis: 26 Years’ Experience with Low-Dose Long-Term Treatment. J. Eur. Acad. Derm. Venereol. 2000, 14, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Apremilast: A Review in Psoriasis and Psoriatic Arthritis. Drugs 2015, 75, 1393–1403. [Google Scholar] [CrossRef]
- Lee, C.S.; Li, K. A Review of Acitretin for the Treatment of Psoriasis. Expert Opin. Drug Saf. 2009, 8, 769–779. [Google Scholar] [CrossRef]
- Russell, G.; Graveley, R.; Seid, J.; Al-Humidan, A.-K.; Skjodt, H. Mechanisms of Action of Cyclosporine and Effects on Connective Tissues. Semin. Arthritis Rheum. 1992, 21, 16–22. [Google Scholar] [CrossRef]
- Canafax, D.M.; Ascher, N.L. Cyclosporine Immunosuppression. Clin. Pharm. 1983, 2, 515–524. [Google Scholar] [CrossRef]
- Stern, R.S. The Risk of Squamous Cell and Basal Cell Cancer Associated with Psoralen and Ultraviolet A Therapy: A 30-Year Prospective Study. J. Am. Acad. Dermatol. 2012, 66, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, S.; Jain, A.; Chopra, D.K.; Khan, W. Psoralen Loaded Liposomal Nanocarriers for Improved Skin Penetration and Efficacy of Topical PUVA in Psoriasis. Eur. J. Pharm. Sci. 2017, 96, 515–529. [Google Scholar] [CrossRef]
- Walunj, M.; Doppalapudi, S.; Bulbake, U.; Khan, W. Preparation, Characterization, and in Vivo Evaluation of Cyclosporine Cationic Liposomes for the Treatment of Psoriasis. J. Liposome Res. 2020, 30, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Dogra, S.; Amarji, B.; Singh, B.; Kumar, S.; Sharma, S.K.; Vinay, K.; Mahajan, R.; Katare, O.P. Efficacy of Novel Topical Liposomal Formulation of Cyclosporine in Mild to Moderate Stable Plaque Psoriasis: A Randomized Clinical Trial. JAMA Dermatol. 2016, 152, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Fathalla, D.; Youssef, E.M.K.; Soliman, G.M. Liposomal and Ethosomal Gels for the Topical Delivery of Anthralin: Preparation, Comparative Evaluation and Clinical Assessment in Psoriatic Patients. Pharmaceutics 2020, 12, 446. [Google Scholar] [CrossRef]
- Pradhan, M.; Singh, D.; Singh, M.R. Novel Colloidal Carriers for Psoriasis: Current Issues, Mechanistic Insight and Novel Delivery Approaches. J. Control. Release 2013, 170, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Iqubal, M.K.; Garg, S.; Ali, J.; Baboota, S. Trends in Nanotechnology-Based Delivery Systems for Dermal Targeting of Drugs: An Enticing Approach to Offset Psoriasis. Expert Opin. Drug Deliv. 2020, 17, 817–838. [Google Scholar] [CrossRef]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid Nanocarriers as Skin Drug Delivery Systems: Properties, Mechanisms of Skin Interactions and Medical Applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.M.; Salah, M.; Rafea, M.; Saleh, N. Liposomal Methotrexate Hydrogel for Treatment of Localized Psoriasis: Preparation, Characterization and Laser Targeting. Med. Sci. Monit. 2008, 14, PI66–PI74. [Google Scholar]
- Agarwal, R.; Saraswat, A.; Kaur, I.; Katare, O.P.; Kumar, B. A Novelliposomal Formulation of Dithranol for Psoriasis: Preliminary Results. J. Derm. 2002, 29, 529–532. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Zhang, Y.; Zhou, X.K.; Yang, H.S.; Chen, X.C.; Wang, Y.S.; Wei, Y.Q.; Chen, L.J.; Hu, H.Z.; et al. Gene Therapy for Psoriasis in the K14-VEGF Transgenic Mouse Model by Topical Transdermal Delivery of Interleukin-4 Using Ultradeformable Cationic Liposome. J. Gene Med. 2010, 12, 481–490. [Google Scholar] [CrossRef]
- Trotta, M.; Peira, E.; Debernardi, F.; Gallarate, M. Elastic Liposomes for Skin Delivery of Dipotassium Glycyrrhizinate. Int. J. Pharm. 2002, 241, 319–327. [Google Scholar] [CrossRef]
- Wang, W.; Shu, G.-F.; Lu, K.-J.; Xu, X.-L.; Sun, M.-C.; Qi, J.; Huang, Q.-L.; Tan, W.-Q.; Du, Y.-Z. Flexible Liposomal Gel Dual-Loaded with All-Trans Retinoic Acid and Betamethasone for Enhanced Therapeutic Efficiency of Psoriasis. J. Nanobiotechnol. 2020, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; Singh, B.; Sharma, G.; Raza, K.; Katare, O.P. Liposomal Fusidic Acid as a Potential Delivery System: A New Paradigm in the Treatment of Chronic Plaque Psoriasis. Drug Deliv. 2016, 23, 1204–1213. [Google Scholar] [CrossRef] [Green Version]
- Srisuk, P.; Thongnopnua, P.; Raktanonchai, U.; Kanokpanont, S. Physico-Chemical Characteristics of Methotrexate-Entrapped Oleic Acid-Containing Deformable Liposomes for in Vitro Transepidermal Delivery Targeting Psoriasis Treatment. Int. J. Pharm. 2012, 427, 426–434. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Shen, L.-N.; Wu, Z.-H.; Zhao, J.-H.; Feng, N.-P. Comparison of Ethosomes and Liposomes for Skin Delivery of Psoralen for Psoriasis Therapy. Int. J. Pharm. 2014, 471, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.Ø.; Rønholt, S.; Salte, R.D.; Jorgensen, L.; Thormann, T.; Basse, L.H.; Hansen, J.; Frokjaer, S.; Foged, C. Calcipotriol Delivery into the Skin with PEGylated Liposomes. Eur. J. Pharm. Biopharm. 2012, 81, 532–539. [Google Scholar] [CrossRef]
- Jatwani, S.; Goyal, A. Vasculitis; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Chang, C.-J.; Ko, Y.-S.; Ko, P.-J.; Hsu, L.-A.; Chen, C.-F.; Yang, C.-W.; Hsu, T.-S.; Pang, J.-H.S. Thrombosed Arteriovenous Fistula for Hemodialysis Access Is Characterized by a Marked Inflammatory Activity. Kidney Int. 2005, 68, 1312–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galkina, E.; Ley, K. Immune and Inflammatory Mechanisms of Atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Galea, R.; Nel, H.J.; Talekar, M.; Liu, X.; Ooi, J.D.; Huynh, M.; Hadjigol, S.; Robson, K.J.; Ting, Y.T.; Cole, S.; et al. PD-L1– and Calcitriol-Dependent Liposomal Antigen-Specific Regulation of Systemic Inflammatory Autoimmune Disease. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Bergheanu, S.C.; Bodde, M.C.; Jukema, J.W. Pathophysiology and Treatment of Atherosclerosis. Neth. Heart J. 2017, 25, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Jongsma, H.; Bekken, J.; Ayez, N.; Hoogewerf, C.J.; Van Weel, V.; Fioole, B. Angioplasty versus Stenting for Iliac Artery Lesions. Cochrane Database Syst. Rev. 2020, 12, CD007561. [Google Scholar] [CrossRef]
- Cho, B.H.S.; Park, J.-R.; Nakamura, M.T.; Odintsov, B.M.; Wallig, M.A.; Chung, B.-H. Synthetic Dimyristoylphosphatidylcholine Liposomes Assimilating into High-Density Lipoprotein Promote Regression of Atherosclerotic Lesions in Cholesterol-Fed Rabbits. Exp. Biol. Med. 2010, 235, 1194–1203. [Google Scholar] [CrossRef]
- Alving, C.R.; Swartz, G.M.; Wassef, N.M.; Ribas, J.L.; Herderick, E.E.; Virmani, R.; Kolodgie, F.D.; Matyas, G.R.; Cornhill, J.F. Immunization with Cholesterol-Rich Liposomes Induces Anti-Cholesterol Antibodies and Reduces Diet-Induced Hypercholesterolemia and Plaque Formation. J. Lab. Clin. Med. 1996, 127, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Lobatto, M.E.; Fayad, Z.A.; Silvera, S.; Vucic, E.; Calcagno, C.; Mani, V.; Dickson, S.D.; Nicolay, K.; Banciu, M.; Schiffelers, R.M.; et al. Multimodal Clinical Imaging to Longitudinally Assess a Nanomedical Anti-Inflammatory Treatment in Experimental Atherosclerosis. Mol. Pharm. 2010, 7, 2020–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Valk, F.M.; van Wijk, D.F.; Lobatto, M.E.; Verberne, H.J.; Storm, G.; Willems, M.C.M.; Legemate, D.A.; Nederveen, A.J.; Calcagno, C.; Mani, V.; et al. Prednisolone-Containing Liposomes Accumulate in Human Atherosclerotic Macrophages upon Intravenous Administration. Nanomedicine 2015, 11, 1039–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duivenvoorden, R.; Tang, J.; Cormode, D.P.; Mieszawska, A.J.; Izquierdo-Garcia, D.; Ozcan, C.; Otten, M.J.; Zaidi, N.; Lobatto, M.E.; van Rijs, S.M.; et al. A Statin-Loaded Reconstituted High-Density Lipoprotein Nanoparticle Inhibits Atherosclerotic Plaque Inflammation. Nat. Commun. 2014, 5, 3065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhaorigetu, S.; Walton, B.; Rodriguez-Aguayo, C.; Lopez-Berestein, G. Delivery of Liposomal Fatty Acid Binding Protein 4 Sirna for the Treatment of Atherosclerosis in Apolipoprotein E-deficient Mice. J. Am. Coll. Cardiol. 2014, 63, A2118. [Google Scholar] [CrossRef] [Green Version]
- Herbst, S.M.; Klegerman, M.E.; Kim, H.; Qi, J.; Shelat, H.; Wassler, M.; Moody, M.R.; Yang, C.-M.; Ge, X.; Zou, Y.; et al. Delivery of Stem Cells to Porcine Arterial Wall with Echogenic Liposomes Conjugated to Antibodies against CD34 and Intercellular Adhesion Molecule-1. Mol. Pharm. 2010, 7, 3–11. [Google Scholar] [CrossRef]
- Duque, J.C.; Tabbara, M.; Martinez, L.; Cardona, J.; Vazquez-Padron, R.I.; Salman, L.H. Dialysis Arteriovenous Fistula Failure and Angioplasty: Intimal Hyperplasia and Other Causes of Access Failure. Am. J. Kidney Dis. 2017, 69, 147–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezhaeva, T.; de Vries, M.R.; Geelhoed, W.J.; van der Veer, E.P.; Versteeg, S.; van Alem, C.M.A.; Voorzaat, B.M.; Eijkelkamp, N.; van der Bogt, K.E.; Agoulnik, A.I.; et al. Relaxin Receptor Deficiency Promotes Vascular Inflammation and Impairs Outward Remodeling in Arteriovenous Fistulas. FASEB J. 2018, fj201800437R. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.-Y.; de Vries, M.R.; Wang, Y.; van der Vorst, J.R.; Vahrmeijer, A.L.; van Zonneveld, A.J.; Roy-Chaudhury, P.; Rabelink, T.J.; Quax, P.H.A.; Rotmans, J.I. Vascular Remodeling and Intimal Hyperplasia in a Novel Murine Model of Arteriovenous Fistula Failure. J. Vasc. Surg. 2014, 59, 192–201.e1. [Google Scholar] [CrossRef] [Green Version]
- Roy-Chaudhury, P.; Wang, Y.; Krishnamoorthy, M.; Zhang, J.; Banerjee, R.; Munda, R.; Heffelfinger, S.; Arend, L. Cellular Phenotypes in Human Stenotic Lesions from Haemodialysis Vascular Access. Nephrol. Dial. Transplant. 2009, 24, 2786–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.; Bezhaeva, T.; Rothuizen, T.C.; Metselaar, J.M.; de Vries, M.R.; Verbeek, F.P.R.; Vahrmeijer, A.L.; Wezel, A.; van Zonneveld, A.-J.; Rabelink, T.J.; et al. Liposomal Prednisolone Inhibits Vascular Inflammation and Enhances Venous Outward Remodeling in a Murine Arteriovenous Fistula Model. Sci. Rep. 2016, 6, 30439. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, H.; Li, Y.; Kanellakis, P.; Tay, C.; Cao, A.; Tipping, P.; Bobik, A.; Toh, B.-H.; Kyaw, T. Phosphatidylserine Liposomes Mimic Apoptotic Cells to Attenuate Atherosclerosis by Expanding Polyreactive IgM Producing B1a Lymphocytes. Cardiovasc. Res. 2015, 106, 443–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benne, N.; van Duijn, J.; Lozano Vigario, F.; Leboux, R.J.T.; van Veelen, P.; Kuiper, J.; Jiskoot, W.; Slütter, B. Anionic 1,2-Distearoyl-Sn-Glycero-3-Phosphoglycerol (DSPG) Liposomes Induce Antigen-Specific Regulatory T Cells and Prevent Atherosclerosis in Mice. J. Control. Release 2018, 291, 135–146. [Google Scholar] [CrossRef]
- Influence of Cholesterol Liposome Immunisation and Immunostimulation on Rabbits Atherosclerosis Induced by a High Cholesterol Diet. Available online: https://www.medscimonit.com/download/index/idArt/502387 (accessed on 28 April 2021).
- Hitchcock, K.E.; Caudell, D.N.; Sutton, J.T.; Klegerman, M.E.; Vela, D.; Pyne-Geithman, G.J.; Abruzzo, T.; Cyr, P.E.P.; Geng, Y.-J.; McPherson, D.D.; et al. Ultrasound-Enhanced Delivery of Targeted Echogenic Liposomes in a Novel Ex Vivo Mouse Aorta Model. J. Control. Release 2010, 144, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Hood, E.D.; Greineder, C.F.; Dodia, C.; Han, J.; Mesaros, C.; Shuvaev, V.V.; Blair, I.A.; Fisher, A.B.; Muzykantov, V.R. Antioxidant Protection by PECAM-Targeted Delivery of a Novel NADPH-Oxidase Inhibitor to the Endothelium in Vitro and in Vivo. J. Control. Release 2012, 163, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voorzaat, B.M.; van der Bogt, K.E.A.; Bezhaeva, T.; van Schaik, J.; Eefting, D.; van der Putten, K.; van Nieuwenhuizen, R.C.; Groeneveld, J.O.; Hoogeveen, E.K.; van der Meer, I.M.; et al. A Randomized Trial of Liposomal Prednisolone (LIPMAT) to Enhance Radiocephalic Fistula Maturation: A Pilot Study. Kidney Int. Rep. 2020, 5, 1327–1332. [Google Scholar] [CrossRef]
- van der Valk, F.M.; Schulte, D.M.; Meiler, S.; Tang, J.; Zheng, K.H.; Van den Bossche, J.; Seijkens, T.; Laudes, M.; de Winther, M.; Lutgens, E.; et al. Liposomal Prednisolone Promotes Macrophage Lipotoxicity in Experimental Atherosclerosis. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1463–1470. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Lintermans, L.L.; Morselt, H.W.M.; Leus, N.G.J.; Ruiters, M.H.J.; Molema, G.; Kamps, J.A.A.M. Anti-VCAM-1 and Anti-E-Selectin SAINT-O-Somes for Selective Delivery of SiRNA into Inflammation-Activated Primary Endothelial Cells. Mol. Pharm. 2013, 10, 3033–3044. [Google Scholar] [CrossRef]
- Chono, S.; Tauchi, Y.; Deguchi, Y.; Morimoto, K. Efficient Drug Delivery to Atherosclerotic Lesions and the Antiatherosclerotic Effect by Dexamethasone Incorporated into Liposomes in Atherogenic Mice. J. Drug Target. 2005, 13, 267–276. [Google Scholar] [CrossRef]
- Banai, S.; Finkelstein, A.; Almagor, Y.; Assali, A.; Hasin, Y.; Rosenschein, U.; Apruzzese, P.; Lansky, A.J.; Kume, T.; Edelman, E.R. Targeted Anti-Inflammatory Systemic Therapy for Restenosis: The Biorest Liposomal Alendronate with Stenting STudy (BLAST)-a Double Blind, Randomized Clinical Trial. Am. Heart J. 2013, 165, 234–240.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pont, I.; Calatayud-Pascual, A.; López-Castellano, A.; Albelda, E.P.; García-España, E.; Martí-Bonmatí, L.; Frias, J.C.; Albelda, M.T. Anti-Angiogenic Drug Loaded Liposomes: Nanotherapy for Early Atherosclerotic Lesions in Mice. PLoS ONE 2018, 13, e0190540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezinover, D.; Saner, F. Organ Transplantation in the Modern Era. BMC Anesth. 2019, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, P.; Cross, N.B.; Barnett, A.N.R.; Palmer, S.C.; Webster, A.C. Polyclonal and Monoclonal Antibodies for Induction Therapy in Kidney Transplant Recipients. Cochrane Database Syst. Rev. 2017, 1, CD004759. [Google Scholar] [CrossRef]
- Penninga, L.; Møller, C.H.; Penninga, E.I.; Iversen, M.; Gluud, C.; Steinbrüchel, D.A. Antibody Induction Therapy for Lung Transplant Recipients. Cochrane Database Syst Rev. 2013, CD008927. [Google Scholar] [CrossRef] [Green Version]
- Aliabadi, A.; Grömmer, M.; Cochrane, A.; Salameh, O.; Zuckermann, A. Induction Therapy in Heart Transplantation: Where Are We Now? Transplant. Int. 2013, 26, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Eisen, H.; Ross, H. Optimizing the Immunosuppressive Regimen in Heart Transplantation. J. Heart Lung Transplant. 2004, 23, S207–S213. [Google Scholar] [CrossRef]
- Opelz, G. Effect of the Maintenance Immunosuppressive Drug Regimen on Kidney Transplant Outcome. Transplantation 1994, 58, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.; Smith, J.M.; Skeans, M.A.; Gustafson, S.K.; Stewart, D.E.; Cherikh, W.S.; Wainright, J.L.; Kucheryavaya, A.; Woodbury, M.; Snyder, J.J.; et al. OPTN/SRTR 2015 Annual Data Report: Kidney. Am. J. Transplant. 2017, 17, 21–116. [Google Scholar] [CrossRef]
- Ganji, M.-R.; Broumand, B. Acute Cellular Rejection. Iran. J. Kidney Dis. 2007, 1, 54–56. [Google Scholar] [PubMed]
- Garces, J.C.; Giusti, S.; Staffeld-Coit, C.; Bohorquez, H.; Cohen, A.J.; Loss, G.E. Antibody-Mediated Rejection: A Review. Ochsner J. 2017, 17, 46–55. [Google Scholar]
- Ali, H.; Soliman, K.; Daoud, A.; Elsayed, I.; Fülöp, T.; Sharma, A.; Halawa, A. Relationship between Rabbit Anti-Thymocyte Globulin and Development of PTLD and Its Aggressive Form in Renal Transplant Population. Ren. Fail. 2020, 42, 489–494. [Google Scholar] [CrossRef]
- Freise, C.E.; Liu, T.; Hong, K.; Osorio, R.W.; Papahadjopoulos, D.; Ferrell, L.; Ascher, N.L.; Roberts, J.P. The Increased Efficacy and Decreased Nephrotoxicity of a Cyclosporine Liposome. Transplantation 1994, 57, 928–932. [Google Scholar] [CrossRef]
- Binder, J.; Braeutigam, R.; Oertl, A.; Kramer, W.; Jonas, D.; Hancock, W.W.; Kupiec-Weglinski, J.W. Methylprednisolone in Bilayer Liposomes Prolongs Cardiac and Renal Allograft Survival, Inhibits Macrophage Activation, and Selectively Modifies Antigen Presentation and T-Helper Cell Function in Rat Recipients. Transplant. Proc. 1998, 30, 1051. [Google Scholar] [CrossRef]
- Mishina, E.V.; Binder, J.; Kupiec-Weglinski, J.W.; Jusko, W.J. Effect of Liposomal Methylprednisolone on Heart Allograft Survival and Immune Function in Rats. J. Pharmacol. Exp. Ther. 1994, 271, 868–874. [Google Scholar] [PubMed]
- Czigany, Z.; Lurje, I.; Schmelzle, M.; Schöning, W.; Öllinger, R.; Raschzok, N.; Sauer, I.M.; Tacke, F.; Strnad, P.; Trautwein, C.; et al. Ischemia-Reperfusion Injury in Marginal Liver Grafts and the Role of Hypothermic Machine Perfusion: Molecular Mechanisms and Clinical Implications. J. Clin. Med. 2020, 9, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brüggenwirth, I.M.A.; Martins, P.N. RNA Interference Therapeutics in Organ Transplantation: The Dawn of a New Era. Am. J. Transplant. 2020, 20, 931–941. [Google Scholar] [CrossRef]
- Gillooly, A.R.; Perry, J.; Martins, P.N. First Report of SiRNA Uptake (for RNA Interference) During Ex Vivo Hypothermic and Normothermic Liver Machine Perfusion. Transplantation 2019, 103, e56–e57. [Google Scholar] [CrossRef]
- Alemdar, A.Y.; Sadi, D.; Mcalister, V.C.; Mendez, I. Liposomal Formulations of Tacrolimus and Rapamycin Increase Graft Survival and Fiber Outgrowth of Dopaminergic Grafts. Cell Transplant. 2004, 13, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Steiner, R.W.; Awdishu, L. Steroids in Kidney Transplant Patients. Semin. Immunopathol. 2011, 33, 157–167. [Google Scholar] [CrossRef] [Green Version]
- van Alem, C.M.A.; Schmidbauer, M.; Rong, S.; Derlin, K.; Schmitz, J.; Bräsen, J.H.; Thorenz, A.; Chen, R.; Ruben, J.M.; Winter, E.M.; et al. Liposomal Delivery Improves the Efficacy of Prednisolone to Attenuate Renal Inflammation in a Mouse Model of Acute Renal Allograft Rejection. Transplantation 2020, 104, 744–753. [Google Scholar] [CrossRef] [Green Version]
- Sen, L.; Hong, Y.S.; Luo, H.; Cui, G.; Laks, H. Efficiency, Efficacy, and Adverse Effects of Adenovirus vs. Liposome-Mediated Gene Therapy in Cardiac Allografts. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1433–H1441. [Google Scholar] [CrossRef]
- Iacono, A.; Wijesinha, M.; Rajagopal, K.; Murdock, N.; Timofte, I.; Griffith, B.; Terrin, M. A Randomised Single-Centre Trial of Inhaled Liposomal Cyclosporine for Bronchiolitis Obliterans Syndrome Post-Lung Transplantation. ERJ Open Res. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, K.; Takeda, S.; Miyoshi, S.; Minami, M.; Nakane, S.; Ohta, M.; Sawa, Y.; Matsuda, H. Application of HVJ-Liposome Mediated Gene Transfer in Lung Transplantation-Distribution and Transfection Efficiency in the Lung. Eur. J. Cardiothorac. Surg. 2005, 27, 768–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Lu, Y.; Lian, Y.; Chen, Z.; Xia, J.; Meng, L.; Qi, Z. Macrophage Depletion Improves Chronic Rejection in Rats with Allograft Heart Transplantation. Transplant. Proc. 2020, 52, 992–1000. [Google Scholar] [CrossRef]
- Alemdar, A.Y.; Sadi, D.; McAlister, V.; Mendez, I. Intracerebral Co-Transplantation of Liposomal Tacrolimus Improves Xenograft Survival and Reduces Graft Rejection in the Hemiparkinsonian Rat. Neuroscience 2007, 146, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Boudinot, F.D.; Jusko, W.J. Dose-Dependent Pharmacokinetics of Prednisolone in Normal and Adrenalectomized Rats. J. Pharmacokinet. Biopharm. 1986, 14, 453–467. [Google Scholar] [CrossRef]
- Rose, J.Q.; Yurchak, A.M.; Jusko, W.J. Dose Dependent Pharmacokinetics of Prednisone and Prednisolone in Man. J. Pharmacokinet. Biopharm. 1981, 9, 389–417. [Google Scholar] [CrossRef] [PubMed]
- Lo Sasso, G.; Schlage, W.K.; Boué, S.; Veljkovic, E.; Peitsch, M.C.; Hoeng, J. The Apoe(-/-) Mouse Model: A Suitable Model to Study Cardiovascular and Respiratory Diseases in the Context of Cigarette Smoke Exposure and Harm Reduction. J. Transl. Med. 2016, 14, 146. [Google Scholar] [CrossRef] [Green Version]
- Schuett, K.A.; Lehrke, M.; Marx, N.; Burgmaier, M. High-Risk Cardiovascular Patients: Clinical Features, Comorbidities, and Interconnecting Mechanisms. Front. Immunol. 2015, 6, 591. [Google Scholar] [CrossRef] [Green Version]
- Fogel, D.B. Factors Associated with Clinical Trials That Fail and Opportunities for Improving the Likelihood of Success: A Review. Contemp. Clin. Trials Commun. 2018, 11, 156–164. [Google Scholar] [CrossRef]
- BIO Industry Analysis. Clinical Development Success Rates 2006–2015; BIO: Washington, DC, USA, 2016. [Google Scholar]
- Satalkar, P.; Elger, B.S.; Hunziker, P.; Shaw, D. Challenges of Clinical Translation in Nanomedicine: A Qualitative Study. Nanomedicine 2016, 12, 893–900. [Google Scholar] [CrossRef] [Green Version]
- Dézsi, L.; Fülöp, T.; Mészáros, T.; Szénási, G.; Urbanics, R.; Vázsonyi, C.; Őrfi, E.; Rosivall, L.; Nemes, R.; Kok, R.J.; et al. Features of Complement Activation-Related Pseudoallergy to Liposomes with Different Surface Charge and PEGylation: Comparison of the Porcine and Rat Responses. J. Control. Release 2014, 195, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Simberg, D.; González-Fernández, Á.; Barenholz, Y.; Dobrovolskaia, M.A. Roadmap and Strategy for Overcoming Infusion Reactions to Nanomedicines. Nat. Nanotechnol. 2018, 13, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Metselaar, J.M.; Lammers, T. Challenges in Nanomedicine Clinical Translation. Drug Deliv. Transl. Res. 2020, 10, 721–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuni, A.M.; Lipman, A.; Barenholz, Y. Damage to Liposomal Lipids: Protection by Antioxidants and Cholesterol-Mediated Dehydration. Chem. Phys. Lipids 2000, 105, 121–134. [Google Scholar] [CrossRef]
- Barrera, P.; Mulder, S.; Smetsers, A.; Storm, G.; Beijnen, J.; Metselaar, J. Long-Circulating Liposomal Prednisolone versus Pulse Intramuscular Methylprednisolone in Patients with Active Rheumatoid Arthritis. Arthritis Rheum. 2008, 58, 3976–3977. [Google Scholar]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Goldys, E.M.; Deng, W. Light-Induced Liposomes for Cancer Therapeutics. Prog. Lipid Res. 2020, 79, 101052. [Google Scholar] [CrossRef]
- Li, C.; Han, X. Melanoma Cancer Immunotherapy Using PD-L1 SiRNA and Imatinib Promotes Cancer-Immunity Cycle. Pharm. Res. 2020, 37, 109. [Google Scholar] [CrossRef]
- Du, Y.; Liang, X.; Li, Y.; Sun, T.; Jin, Z.; Xue, H.; Tian, J. Nuclear and Fluorescent Labeled PD-1-Liposome-DOX-64Cu/IRDye800CW Allows Improved Breast Tumor Targeted Imaging and Therapy. Mol. Pharm. 2017, 14, 3978–3986. [Google Scholar] [CrossRef] [PubMed]


| Drug Class | Drug | Animal | Model | Route | Effect * | Ref. |
|---|---|---|---|---|---|---|
| Glucocorticoid | Prednisolone phosphate in PEGylated liposomes | Mouse | Antigen-induced arthritis | I.V. * | Effective inhibition of inflammation and potential reduced bone erosion | [94] |
| Glucocorticoid | Dexamethasone phosphate in liposomes | Mouse | Collagen-induced arthritis | I.V. | Persistent anti-inflammatory effect, limitation of the suppression of the HPA axis, absence of the drug-induced gluconeogenesis | [95] |
| Glucocorticoid | Methyl prednisolone hemisuccinate in pegylated nanoliposomes | Rat and Beagle | Adjuvant-induced arthritis | I.V. | Superior therapeutic efficacy to free glucocorticoid | [96] |
| DMARD * | Prodrug of sulfapyridine in liposomes | Rat | Adjuvant-induced arthritis | I.A.* | Reverse the symptoms ofinflammation | [93] |
| DMARD | Sinomenine hydrochloride in thermosensitive liposomes | Rat | Adjuvant-induced arthritis | I.V. | Superior antirheumatoid arthritis effect | [85] |
| DMARD | Methotrexate and indocyanine green loaded iRGD peptide-functionalized echogenic liposomes | Mouse | Collagen-induced arthritis | I.V. | Greatly improved therapeutic efficacy and reduced methotrexate side effects, allows for ultrasound mediated release | [87] |
| DMARD | Dimeric artesunate phospholipid conjugate in liposomes | Rat | Adjuvant-induced arthritis | I.V. | Significantly higher inhibition of the cell secretion of proinflammatory cytokines | [91] |
| NABD * | TNF-α small interfering RNA wrapsome vs. control si-RNA | Mouse | Collagen-induced arthritis | I.V. | Significant decreases in severity of arthritis and TNF-α mRNA | [97] |
| NABD | anti-IL-1, anti-IL-6, or anti-IL-18 small interfering RNA in lipoplexes vs. anti-TNF siRNA lipoplex-based treatment | Mouse | Collagen-induced arthritis | I.V. | Significantly reduced the incidence and severity of arthritis | [98] |
| NABD | TNF-α small interfering RNA in a cationic liposome formulation vs. untreated | Mouse | Collagen-induced arthritis | I.P.* | Inhibition (50–70%) of articular and systemic TNF secretion | [99] |
| Hybrid | Dexamethasone sodium phosphate in Folate conjugated PEG liposomes | Rat | Collagen-induced arthritis | I.V. | Improved drug release under ultrasound vs. unstimulated liposomes | [100] |
| Hybrid | Prednisolone and Methotrexate in PEGylated liposomes | Rat | Carrageenan induced arthritis | I.V. | Higher inhibition of edema and increased local accumulation of double liposomes compared to single layer liposomes | [89] |
| Hybrid | Prednisolone phosphate and Fludeoxyglucose (18 F) in PEGylated liposomes vs. untreated | Mouse | Antigen-induced arthritis | I.V. | Increased uptake in inflamed joints, suppression of joint swelling after treatment | [101] |
| Hybrid | Dexamethasone in Sialic Acid Conjugate–Modified Liposomes in a size range | Rat | Adjuvant-induced arthritis | I.V. | Neutrophil targeting was achieved with small liposomes, anti-RA efficacy was established | [102] |
| Hybrid | Dexamethasone palmitate in Sialic Acid Conjugate–Modified Liposomes | Rat | Adjuvant-induced arthritis | I.V. | Accumulation in peripheral blood neutrophils, strong anti-inflammatory effect | [103] |
| Hybrid | Dexamethasone in liposomes with a novel peptide ligand ART-2 | Rat | Antigen-induced arthritis | S.C. * | Enhanced endothelial cell-binding, increased efficacy compared to free drugs | [104] |
| Hybrid | Betamethasone in folate-conjugated liposomes vs. untargeted liposomes | Rat | Adjuvant-induced arthritis | I.V. | Less paw swelling, lower arthritis scores, a reduction in bone erosion, less splenomegaly and better maintenance of body weight | [105] |
| Hybrid | Prednisolone in peptide targeted liposomes | Rat | Adjuvant-induced arthritis | I.V. | Local accumulation vs. unaffected joints, and minimal inflammation in vivo | [106] |
| Hybrid | Methotrexate and catalase co-encapsulated in folate and PEG conjugated liposomes | Mouse | Collagen-induced arthritis | I.V. | Enhanced accumulation, reinforced therapeutic efficacy and minimal toxicity | [88] |
| Hybrid | Methotrexate in Folate Tagged Liposomes | Mouse | Collagen-induced arthritis | I.P. | Highly specific and efficient in targeting folate receptor β, and also significantly increase the clinical benefit | [107] |
| Hybrid | NF-κB decoy oligodeoxynucleotides, gold nanorods, and dexamethasone in folate modified liposomes | Mouse | Adjuvant-induced arthritis | I.V. | Significantly enhanced anti-inflammatory efficacy | [108] |
| Hybrid | NF-kB small interfering RNA and methothrexate in calcium phosphate/liposome | Mouse | Collagen-induced arthritis | I.V. | Effectively blocked the NF-kB signaling pathway, reduced the expression of proinflammatory cytokines | [90] |
| Hybrid | NF-kB targeted siRNA and methotrexate in a calcium phosphate/liposome-based hybrid nanocarrier | Mouse | Collagen-induced arthritis | I.D. * | Significant suppression of arthritis progression, targeting of macrophages, no decreased lymphocyte count | [90] |
| Hybrid | IL-27 liposomes coated with peptide ligand ART-1 | Rat | Antigen-induced arthritis | I.V. | Better binding to endothelial cells, effective in suppressing disease progression, improved safety profile | [109] |
| Hybrid | Naringin, sulforaphane, and phenethyl isothiocyanate | Rat | Adjuvant-induced arthritis | I.V. | Antiarthritic activity observed after treatment with nutraceuticals | [92] |
| Hybrid | APO2L/TRAIL bound to liposomes | Rabbit | Adjuvant-induced arthritis | I.A. | Increased bioactivity compared to unmodified liposomes | [110] |
| Route | Drug | Animal | Model | Challenge | Effect * | Ref. |
|---|---|---|---|---|---|---|
| Topical biologics | IL-17 Receptor targeting Liposomal Spherical Nucleic Acids vs. scrambled L-SNA | In vitro human skin | Healthy skin culture | Poor transdermal delivery | Reduced the expression of IL17RA by 72% | [38] |
| Topical biologics | IL-17 Receptor targeting Liposomal Spherical Nucleic Acids vs. scrambled L-SNA | Mouse | Imiquimod induced psoriatic plaque | Poor transdermal delivery | Reversed the development of psoriasis | [38] |
| Topical biologics | Liposomes containing plasmids with murine IL-4 gene | Mouse | K14-VEGF transgenic mice with moderate psoriasis | Topical transdermal gene delivery | Plasmid DNA was transdermally delivered and antipsoriatic efficacy was observed compared to untreated mice | [131] |
| Topical calcineurin inhibitor | Cyclosporine in liposomes vs. untreated animals | Mouse | Imiquimod induced psoriatic plaque | Adverse effects in systemic exposure, low topical absorption | Psoriatic features are markedly reduced after treatment | [123] |
| Topical calcineurin inhibitor | Cyclosporine in a liposomal formulation | Human | Psoriatic patients (n = 38) | Poor transdermal delivery | Treatment with cyclosporine lipogel resulted in complete clearance in 41% of psoriasis lesional sites in a safe manner; future efficacy studies are required | [124] |
| Topical keratolytic | Dipotassium glycyrrhizinate in elastic liposomes | Pig | Ex vivo porcine skin | Poor transdermal delivery | Elastic liposomes able to penetrate through membrane pores of diameter much smaller than their own diameter, and skin deposition increased 4.5-fold compared with aqueous solutions | [132] |
| Topical keratolytic | Dithranol in liposomes | Human | Psoriatic patients (n = 9) | Low stability and irritation in topical creams | 5 patients were totally cleared of lesions, and a 50% reduction was achieved in two other patients | [130] |
| Topical keratolytic | Anthralin in liposomal and ethosomal gel | Human | Psoriatic patients | Reduction of side effects and increasing efficacy | No adverse effects were detected, and both formulations increased the efficacy of anthralin, with a significantly higher effect in ethosomes | [125] |
| Topical hybrid | all-trans retinoic acid and betamethasone in flexible liposomes | Mouse | Imiquimod induced psoriatic plaque | Enhanced therapeutic efficiency | Reduced thickness of epidermal layer and the level of proinflammatory cytokines compared to free drugs | [133] |
| Topical corticosteroid | Fusidic acid in liposomes | Mouse | Mouse tail model | Poor transdermal delivery | Increased permeation of the skin and increased efficacy | [134] |
| Topical corticosteroid | Methotrexate in oleic acid-containing deformable liposomes | Pig | Ex vivo porcine skin | Poor transdermal delivery | Liposomes with a size range of 80–140 nm showed enhanced skin permeability; inclusion of oleic acid increased deformability and enhanced permeability | [135] |
| Topical PUVA | Psoralen in liposomes and ethosomes | Rat | In vitro normal rat skin | Poor transdermal delivery | Transdermal flux and skin deposition using ethosomes were 3.50 and 2.15 times those achieved using liposomes, respectively | [136] |
| Topical methotrexate | Methotrexate in liposomes combined with laser targeting | Mouse | Healthy skin | Systemic treatment is limited due to several adverse effects | Treated mice showed no recurrence of psoriasis symptoms | [129] |
| Topical vitamin D analogue | Calcipotriol in PEGylated liposomes | Pig | Ex vivo porcine skin | Poor transdermal delivery | Liposome size affects penetration into the stratum corneum; deposition improved slightly with PEGylated liposomes vs. unPEGylated liposomes | [137] |
| Goal | Liposome Formulation | Animal | Model | Effect * | Ref. |
|---|---|---|---|---|---|
| Cholesterol entrapment | Synthetic dimyristoylphosphatidylcholine liposomes in high-density lipoprotein | Rabbit | Cholesterol-fed rabbits | Significantly decreased aortic cholesterol contents and decreased artherosclerotic plaque volume compared to untreated animals | [145] |
| Vaccination—Activation of atheroprotective peritoneal B1a cells | Phosphatidylserine liposomes vs. control liposomes | Mouse | ApoE-KO mice | Reduction of oxidized LDL in the lesion and reduced necrotic core size | [157] |
| Artherosclerosis vaccine | Anionic 1,2-distearoyl-sn-glycero-3-phosphoglycerol liposomes | Mouse | Western type diet | Induction of antigen-specific Tregs, reduced plaque formation, increased plaque stability | [158] |
| Vaccination—inducing anticholesterol IgG and IgM antibodies | Liposomes containing 71% cholesterol and lipid A as an adjuvant | Rabbit | A diet containing 0.5% to 1.0% cholesterol | Decrease in elevation of serum cholesterol accompanied by reduced antibody levels, indicating antibody mediated decrease; also: decreased artherosclerosis risk and decreased plaque size compared to nonimmunized animals | [146] |
| Vaccination—inducing anticholesterol antibodies | Cholesterol liposomes | Rabbit | High cholesterol diet | Immunization was effective in preventing artherosclerotic plaque formation compared to negative control animals, but this effect was absent upon immunostimulation with a Gram-negative bacterial product | [159] |
| Induction of tolerance in dendritic cells and T cells | Liposomes encapsulating calcitriol, and PD-L1 | In vitro and mice | Goodpasture’s vasculitis model | In vitro induction of Tregs was observed, and the severity of vasculitis was reduced in vivo compared to untreated animals | [142] |
| Evaluate echogenic liposome delivery | Rhodamine-labeled echogenic liposomes | Mouse | Ex vivo aortae from ApoE-deficient mice | Subendothelial delivery of rhodamine liposomes was observed in ultrasound treated aortae but not in untreated samples; no ultrasound-mediated damage was observed | [160] |
| Stem cell delivery and ultrasound guided release | CD34 and ICAM-1 coupled echogenic immunoliposomes | Pig | High cholesterol diet | Stem cells were successfully delivered to the arterial intima, and this effect was enhanced upon ultrasound treatment | [151] |
| Delivery of liposomal si-RNA | Fatty Acid Binding Protein 4 si-RNA in liposomes vs. control si-RNA in liposomes | Mouse | ApoE-deficient mice | Successful delivery of siRNA to artherosclerotic plaques and successful suppression of FABP4 expression | [150] |
| Drug delivery | Statins in a reconstituted high-density lipoprotein nanoparticle carrier | Mouse | ApoE-deficient mice | Inhibition of the inflammatory progression within atherosclerotic plaques | [149] |
| Drug delivery | NADPH oxidase inhibitor in immunoliposomes targeted to endothelial marker platelet endothelial cell adhesion molecule | Mouse | LPS challenged lungs | Alleviation of pathological disruption of endothelial permeability barrier function compared to untreated animals | [161] |
| Drug delivery | Prednisolone phosphate in PEGylated liposomes | Human | Patients receiving an arteriovenous fistula in the forearm | The clinical trial was concluded prematurely due to low inclusion, and no treatment effect was observed | [162] |
| Drug delivery | Prednisolone in liposomes | Mouse | Western type diet | Atherosclerosis was accelerated with increased macrophage content, larger necrotic cores, and more advanced plaque formation compared to empty liposome treatment | [163] |
| Drug delivery | anti-VCAM-1 and anti-E-selectin short interferin RNAs in cationic amphiphile SAINT-C18 liposomes | Human cells and mouse | Human umbilical vein endothelial cells and human aortic endothelial cells, and TNF-α induced mice | Successful delivery to in vitro cultured endothelial cells and subsequent downregulation of target mRNA; in vivo pharmacokinetics were comparable to conventional PEGylated liposomes | [164] |
| Drug delivery | Dexamethasone in liposomes | Mouse | Aortic artherosclerotic lesions | Liposomes of 202 nm diameter had optimal uptake in aortic lesions, allowing for lower dose treatment | [165] |
| Drug delivery to circulating monocytes | Liposomal Alendronate vs. placebo | Human | Patients undergoing bare metal stent implantation | Intravenous administration is safe and effectively modulates monocyte behavior | [166] |
| Drug delivery to atherosclerotic plaque macrophages | Prednisolone in liposomes vs. placebo | Human | Healthy volunteers and patients with atherosclerotic disease | Intravenous injection with liposomal prednisolone led to accumulation in plaque macrophages, but anti-inflammatory efficacy was not observed | [148] |
| Drug delivery | Prednisolone phosphate in PEGylated liposomes | Rabbit | Artherosclerotic plaques induced by high cholesterol diet | Local accumulation in the plaque was achieved and prednisolone treatment was efficient | [147] |
| Drug delivery | Fumagillin-loaded liposomes compared to empty liposomes | Mouse | Apolipoprotein E-knockout (ApoE-KO) mice | Decrease in lesion size | [167] |
| Goal | Liposome Formulation | Animal | Model | Effect * | Ref. |
|---|---|---|---|---|---|
| Gene therapy delivery in heart transplantation | IL-10 gene plasmid in liposomes | Rat | Functional heterotopic heart transplantation | Gene transfer efficiency was lower than the adenovirus group, but the efficacy of liposome mediated transfer was higher | [187] |
| Gene therapy in lung transplantation | Hemagglutinating virus of Japan gene transfer system containing plasmid DNA in liposomes | Rat | Organ perfusion or intratracheal instillation of liposomes during lung transplantation | Low levels of gene transfer to endothelial cells, and moderate transfection to airway and alveolar cells | [189] |
| Drug delivery during chronic rejection | Chlodronate liposomes | Rat | Chronic allograft rejection | Reduced expression of proinflammatory cytokines and reduced T cell proliferation compared to untreated animals | [190] |
| Drug delivery during acute rejection | Prednisolone in PEGylated liposomes | Mouse | Acute cellular rejection after kidney transplantation | Liposomes accumulated in the transplanted kidney and liposomal prednisolone improved the efficacy in attenuating the renal inflammation | [186] |
| Drug delivery on the organ level | Prednisolone in PEGylated liposomes | Rat | Renal ischemia reperfusion damage | Liposomes accumulate locally in the inflamed kidney and seem to extravasate via peritubular capillaries | [56] |
| Drug delivery after lung transplantation | Cyclosporin in liposomes | Human | Lung transplant patients suffering from bronchiolitis obliterans syndrome (BOS) | An increased efficacy on BOS free survival was observed, compared to oral cyclosporin, with no systemic toxicity | [188] |
| Drug delivery during cell graft | Liposomal tacrolimus and rapamycin | Rat | Fetal ventral mesencephalic cell transplantation into the brains of rats with unilateral 6-hydroxydopamine lesions | Higher survival of cell grafts and increased fiber outgrowth after synergistic treatment using tacrolimus and rapamycin compared to separate administration | [184] |
| Drug delivery during xenotransplantation | Tacrolimus in liposomes | Rat | Xenotransplantation of mouse cells into a hemiparkinsonian rat | Increased survival of xenotransplanted cells and a decrease in rotational behavior compared to untreated animals | [191] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Alem, C.M.A.; Metselaar, J.M.; van Kooten, C.; Rotmans, J.I. Recent Advances in Liposomal-Based Anti-Inflammatory Therapy. Pharmaceutics 2021, 13, 1004. https://doi.org/10.3390/pharmaceutics13071004
van Alem CMA, Metselaar JM, van Kooten C, Rotmans JI. Recent Advances in Liposomal-Based Anti-Inflammatory Therapy. Pharmaceutics. 2021; 13(7):1004. https://doi.org/10.3390/pharmaceutics13071004
Chicago/Turabian Stylevan Alem, Carla M. A., Josbert M. Metselaar, Cees van Kooten, and Joris I. Rotmans. 2021. "Recent Advances in Liposomal-Based Anti-Inflammatory Therapy" Pharmaceutics 13, no. 7: 1004. https://doi.org/10.3390/pharmaceutics13071004
APA Stylevan Alem, C. M. A., Metselaar, J. M., van Kooten, C., & Rotmans, J. I. (2021). Recent Advances in Liposomal-Based Anti-Inflammatory Therapy. Pharmaceutics, 13(7), 1004. https://doi.org/10.3390/pharmaceutics13071004