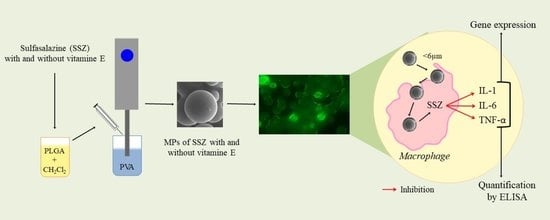
Sulfasalazine Microparticles Targeting Macrophages for the Treatment of Inflammatory Diseases Affecting the Synovial Cavity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Microparticles
2.3. Characterization of Microparticles
2.3.1. Morphological Characterization and Size Distribution of Microparticles
2.3.2. Encapsulation Efficiency and Drug Loading
2.3.3. In Vitro Release Studies
2.4. Cell Culture Studies
2.4.1. Phagocytosis Studies
2.4.2. MTT Cell Viability Assay
2.4.3. Anti-Inflammatory Activity of the MPs Formulations in Cell Cultures
2.4.4. RNA Extraction
2.4.5. Reverse Transcription-Polymerase Chain Reaction
2.4.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fauci, A.S.; Langford, C. Harrison’s Rheumatology, 3rd ed.; McGraw-Hill Education: New York, NY, USA, 2013. [Google Scholar]
- Gabriel, S.E. The epidemiology of rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2001, 27, 269–281. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury: Antirheumatic Agents, Bethesda. 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548204 (accessed on 12 November 2020).
- Köhler, B.M.; Günther, J.; Kaudewitz, D.; Lorenz, H.M. Current therapeutic options in the treatment of rheumatoid arthritis. J. Clin. Med. 2019, 8, 938. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.A.; Saag, K.G.; Bridges, S.L.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2016, 68, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougados., M.; Combe, B.; Cantagrel, A.; Goupille, P.; Olive, P.; Schattenkirchner, M.; Meusser, S.; Paimela, L.; Rau, R.; Zeidler, H.; et al. Combination therapy in early rheumatoid arthritis: A randomised, controlled, double blind 52 week clinical trial of sulphasalazine and methotrexate compared with the single components. Ann. Rheum. Dis. 1999, 58, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, M.A.J.; van Soesbergen, R.M.; Boers, M.; Zwinderman, A.H.; Fiselier, T.J.W.; Franssen, M.J.A.M. Long-term outcome of juvenile idiopathic arthritis following a placebo-controlled trial: Sustained benefits of early sulfasalazine treatment. Ann. Rheum. Dis. 2007, 66, 1518–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, N.G.; Awasthi, S.; Singhal, S.S.; Trent, M.B.; Boor, P.J. The role of glutathione S-transferases as a defense against reactive electrophiles in the blood vessel wall. Toxiol. Appl Pharmacol. 1998, 152, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Liptay, S.; Bachem, M.; Häcker, G.; Adler, G.; Debatin, K.M.; Schmid, R.M. Inhibition of nuclear factor kappa B and induction of apoptosis in T-lymphocytes by sulfasalazine. Br. J. Pharmacol. 1999, 28, 1361–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linares, V.; Alonso, V.; Albina, M.L.; Bellés, M.; Sirvent, J.J.; Domingo, J.L.; Sánchez, D.J. Lipid peroxidation and antioxidant status in kidney and liver of rats treated with sulfasalazine. Toxicology 2009, 25, 152–156. [Google Scholar] [CrossRef] [PubMed]
- DeMichele, J.; Rezaizadeh, H.; Goldstein, J.I. Sulfasalazine crystalluria-induced anuric renal failure. Clin. Gastroentero-Hepatol. 2012, 10, A32. [Google Scholar] [CrossRef]
- Heidari, R.; Taheri, V.; Rahimi, H.R.; Shirazi-Yeganeh, B.; Niknahad, H.; Najibi, A. Sulfasalazine induced renal injury in rats and the protective role of thiol-reductants. Ren. Fail. 2016, 38, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Niknahad, H.; Heidari, R.; Mohammadzadeh, R.; Ommati, M.M.; Khodaei, F.; Azarpira, N.; Abdoli, N.; Zarei, M.; Asadi, B.; Rasti, M.; et al. Sulfasalazine induces mitochondrial dysfunction and renal injury. Ren. Fail. 2017, 39, 745–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, M.; Baron, B. The role of TNF-α in rheumatoid arthritis: A focus on regulatory T cells. J. Clin. Transl. Res. 2016, 2, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Gilding, D.K.; Reed, A.M. Biodegradable polymers for use in surgery -polyglycolic-poly (lactic acid) homopolymers and copolymers: 1. Polymer 1979, 20, 1459–1964. [Google Scholar] [CrossRef]
- Hirota, K.; Terada, H. Endocytosis of particles formulations by macrophages and its application to clinical treatment. In Molecular Regulation of Endocytosis; Ceresa, B., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Patel, B.; Gupta, N.; Ahsan, F. Particle engineering to enhance or lessen particle uptake by alveolar macrophages and to influence the therapeutic outcome. Eur. J. Pharm. Biopharm. 2015, 89, 163–174. [Google Scholar] [CrossRef]
- Pei, Y.; Yeo, Y.J. Drug delivery to macrophages: Challenges and opportunities. J. Control. Release 2016, 240, 202–211. [Google Scholar] [CrossRef]
- Kabir, A.; Furton, K.G.; Tinari, N.; Grossi, L.; Innosa, D.; Macerola, D.; Tartaglia, A.; Di Donato, V.; D’Ovidio, C.; Locatelli, M. Fabric phase sorptive extraction-high performance liquid chromatography-photo diode array detection method for simultaneous monitoring of three inflammatory bowel disease treatment drugs in whole blood, plasma and urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1084, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Wilfinger, W.W.; Mackey, K.; Chomczynski, P. Effect of pH and ionic strength on the spectrophotometric assessment of nucleic acid purity. Biotechniques 1997, 22, 474–481. [Google Scholar] [CrossRef]
- Kinne, R.W.; Stuhlmüller, B.; Burmester, G.R. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis Res. Ther. 2007, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Garapaty, A.; Champion, J.A. Tunable particles alter macrophage uptake based on combinatorial effects of physical properties. Bioeng. Transl. Med. 2017, 2, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Makino, K.; Yamamoto, N.; Higuchi, K.; Harada, N.; Ohshima, H.; Terada, H. Phagocytic uptake of polystyrene microspheres by alveolar macrophages: Effects of the size and surface properties of the microspheres. Colloid Surf. B 2003, 27, 33–39. [Google Scholar] [CrossRef]
- Mathaes, R.; Winter, G.; Besheer, A.; Engert, J. Influence of particle geometry and PEGylation on phagocytosis of particulate carriers. Int. J. Pharm. 2014, 465, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Gupta, S.; Mohammad, S.; Ahmad, I. Development of α-tocopherol surface-modified targeted delivery of 5-fluorouracil-loaded poly-D, L-lactic-co-glycolic acid nanoparticles against oral squamous cell carcinoma. J. Cancer Res. Ther. 2019, 15, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sarfraz, M.K.; Clas, S.D.; Roa, W.; Löbenberg, R. Hyaluronic acid-tocopherol succinate-based self-assembling micelles for targeted delivery of rifampicin to Alveolar Macrophages. J. Biomed. Nanotechnol. 2015, 11, 1312–1329. [Google Scholar] [CrossRef]
- Hirota, K.; Hasegawa, T.; Hinata, H.; Ito, F.; Inagawa, H.; Kochi, C.; Soma, G.; Makino, K.; Terada, H. Optimum conditions for efficient phagocytosis of rifampicin loaded PLGA microspheres by alveolar macrophages. J. Control. Release 2007, 119, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sancho, C.; Herrero-Vanrell, R.; Negro, S. Poly (D,L-lactide-co-glycolide) microspheres for long-term intravitreal delivery of aciclovir: Influence of fatty and non-fatty additives. J. Microencapsul. 2003, 20, 799–810. [Google Scholar] [CrossRef]
- Barcia, E.; Herradón, C.; Herrero-Vanrell, R. Biodegradable additives modulate ganciclovir release rate from PLGA microspheres destined to intraocular administration. Lett. Drug Des. Discov. 2005, 2, 148–149. [Google Scholar] [CrossRef]
- Marcianes, P.; Negro, S.; Barcia, E.; Montejo, C.; Fernandez-Carballido, A. Potential active targeting of gatifloxacin to macrophages by eeans of surface-modified PLGA microparticles destined to treat tuberculosis. AAPS Pharm. Sci. Tech. 2020, 21, 15. [Google Scholar] [CrossRef]
- Misharin, A.; Morales-Nebreda, L.; Mutlu, G.M.; Budinger, G.R.; Perlman, H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir Cell Mol. Biol. 2013, 49, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, A.J.; Pradel, L.C.; Chasson, L.; Van Rooijen, N.; Grau, G.E.; Hunt, N.H.; Chimini, G. Technical Advance: Autofluorescence as a tool for myeloid cell analysis. J. Leukoc. Biol. 2010, 88, 597–603. [Google Scholar] [CrossRef]
- Dinarello, C.A. IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J. Allergy Clin. Immunol. 1999, 103, 11–24. [Google Scholar] [CrossRef]
- Flores, D.; Marquez, J.; Garza, L.; Espinoza, L.R. Reactive arthritis: Newer developments. Rheum. Dis. Clin. N. Am. 2003, 29, 37–59. [Google Scholar] [CrossRef]
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmaol. 2009, 125, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Tang, L.; Zou, P.; Zhang, Y.; Wang, Z.; Fang, Q.; Jiang, L.; Chen, G.; Xu, Z.; Zhang, H.; et al. Synthesis and biological evaluation of allylated and prenylated mono-carbonyl analogs of curcumin as anti-inflammatory agents. Eur. J. Med. Chem. 2014, 74, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, C.C. Inflammatory response of macrophages in infection. Hepatobiliary Pancreat Dis. Int 2014, 13, 138–152. [Google Scholar] [CrossRef]
- Singh, U.; Jialal, I. Anti-inflammatory effects of alpha-tocopherol. Ann. N. Y. Acad. Sci. 2006, 1031, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Lindström, T.M.; Bennett, P.R. The role of nuclear factor kappa B in human labour. Reproduction 2005, 130, 569–581. [Google Scholar] [CrossRef] [PubMed]










| Formulation | SSZ (%) | FITC (%) | α-tocopherol: Polymer |
|---|---|---|---|
| F-AS | 5 | - | - |
| F-ASE | 5 | - | 1:5 |
| F-A0 | - | - | - |
| F-A0E | - | - | 1:5 |
| F-AF | - | 10 | - |
| F-AFE | - | 10 | 1:5 |
| Formulation | Forward | Reverse |
|---|---|---|
| GADPH | 5′-TGAGGCCGGTGCTGAGTATGTCG-3′ | 5′-CCACAGTCTTCTGGGTGGCAGTG-3′ |
| IL-1 | 5′-AGTTGACGGACCCCAAAAGAT-3′ | 5′-GTTGATGTGCTGCTGCGAGA-3′ |
| IL-6 | 5′-CTTCCATCCAGTTGCCTTCTTG-3′ | 5′-AATTAAGCCTCCGACTTGTGAAG-3′ |
| TNF-α | 5′-GATCTCAAAGACAACCAACATGTG-3′ | 5′-CTCCAGCTGGAAGACTCCTCCCAG-3′ |
| Formulation | DL (mg of Compound/100 mg of MPs) | EE% | Mean Particle Size (µm) | |
|---|---|---|---|---|
| SSZ | FICT | |||
| F-AS | 3.02 ± 0.31 | - | 63.50 ± 6.62 | 4.09 ± 0.64 |
| F-ASE | 3.24 ± 0.48 | - | 81.07 ± 11.92 | 3.87 ± 0.21 |
| F-AF | - | 0.11 ± 0.02 | 1.22 ± 0.24 | 3.98 ± 0.91 |
| F-AFE | - | 0.09 ± 0.01 | 1.13 ± 0.16 | 5.36 ± 0.62 |
| Formulation | LPS (μg) | Concentration (pg/mL) | ||
|---|---|---|---|---|
| IL-1 | IL-6 | TNF-α | ||
| Control | - | 22.6 ± 0.13 | 25.3 ± 0.03 | 220.6 ± 0.3 |
| 0.7 | 43.27 ± 2.79 | 47.70 ± 10.46 | 526.08 ± 0.088 | |
| F-A0 | - | 28.9 ± 0.23 | 30.2 ± 0.03 | 308.9 ± 0.45 |
| 0.7 | 36.38 ± 1.63 | 40.35 ± 1.63 | 653.38 ± 3.5 | |
| F-A0E | - | 30.2 ± 0.02 | 29.83 ± 0.02 | 300.2 ± 0.23 |
| 0.7 | 44.20 ± 4.24 | 49.23 ± 11.06 | 600.4 ± 14.13 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa-Hermosilla, M.-C.; Fernández-Carballido, A.; Hurtado, C.; Barcia, E.; Montejo, C.; Alonso, M.; Negro, S. Sulfasalazine Microparticles Targeting Macrophages for the Treatment of Inflammatory Diseases Affecting the Synovial Cavity. Pharmaceutics 2021, 13, 951. https://doi.org/10.3390/pharmaceutics13070951
Villa-Hermosilla M-C, Fernández-Carballido A, Hurtado C, Barcia E, Montejo C, Alonso M, Negro S. Sulfasalazine Microparticles Targeting Macrophages for the Treatment of Inflammatory Diseases Affecting the Synovial Cavity. Pharmaceutics. 2021; 13(7):951. https://doi.org/10.3390/pharmaceutics13070951
Chicago/Turabian StyleVilla-Hermosilla, Monica-Carolina, Ana Fernández-Carballido, Carolina Hurtado, Emilia Barcia, Consuelo Montejo, Mario Alonso, and Sofia Negro. 2021. "Sulfasalazine Microparticles Targeting Macrophages for the Treatment of Inflammatory Diseases Affecting the Synovial Cavity" Pharmaceutics 13, no. 7: 951. https://doi.org/10.3390/pharmaceutics13070951
APA StyleVilla-Hermosilla, M. -C., Fernández-Carballido, A., Hurtado, C., Barcia, E., Montejo, C., Alonso, M., & Negro, S. (2021). Sulfasalazine Microparticles Targeting Macrophages for the Treatment of Inflammatory Diseases Affecting the Synovial Cavity. Pharmaceutics, 13(7), 951. https://doi.org/10.3390/pharmaceutics13070951