Impact of Laser Speed and Drug Particle Size on Selective Laser Sintering 3D Printing of Amorphous Solid Dispersions
Abstract
:1. Introduction
2. Material and Methods Materials
2.1. Materials
2.2. Feedstock Preparation
2.3. Differential Scanning Calorimetry (DSC)
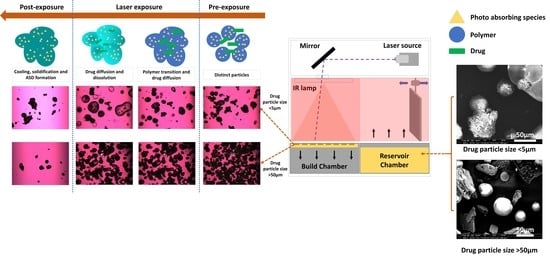
2.4. Selective Laser Sintering (SLS)
2.5. Scanning Electron Microscopy (SEM)
2.6. Hot Stage Microscopy (HSM)
2.7. Powder X-ray Diffraction (PXRD)
2.8. Dynamic Vapor Absorption (DVS)
2.9. In Vitro Drug Release Performance Testing
2.10. Wide-Angle X-ray Scattering (WAXS)
2.11. Particle Size Analysis
3. Results
3.1. SEM and HSM Analysis
3.2. Tablet Morphology and Quality
3.3. Differential Scanning Calorimetry and X-ray Diffraction
3.4. Dynamic Vapor Sorption
3.5. In-Vitro Drug Release Performance Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Aghda, N.H.; Pillai, A.R.; Thakkar, R.; Nokhodchi, A.; Maniruzzaman, M. Emerging 3D printing technologies for drug delivery devices: Current status and future perspective. Adv. Drug Deliv. Rev. 2021, 174, 294–316. [Google Scholar] [CrossRef]
- Aho, J.; Bøtker, J.P.; Genina, N.; Edinger, M.; Arnfast, L.; Rantanen, J. Roadmap to 3D-Printed Oral Pharmaceutical Dosage Forms: Feedstock Filament Properties and Characterization for Fused Deposition Modeling. J. Pharm. Sci. 2019, 108, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Qin, H.; Acevedo, N.C.; Shi, X. Development of methylcellulose-based sustained-release dosage by semisolid extrusion additive manufacturing in drug delivery system. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Conceição, J.; Vaamonde, X.F.; Goyanes, A.; Adeoye, O.; Concheiro, A.; Cabral-Marques, H.; Lobo, J.M.S.; Alvarez-Lorenzo, C. Hydroxypropyl-β-cyclodextrin-based fast dissolving carbamazepine printlets prepared by semisolid extrusion 3D printing. Carbohydr. Polym. 2019, 221, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, M.; Crane, N.B. Binder jetting: A review of process, materials, and methods. Addit. Manuf. 2019, 28, 781–801. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Thakkar, R.; Pillai, A.R.; Wang, J.; Lu, A.; Maniruzzaman, M. Functions of Magnetic Nanoparticles in Selective Laser Sintering (SLS) 3D Printing of Pharmaceutical Dosage Forms. ChemRxiv 2021. [Google Scholar] [CrossRef]
- Kruth, J.; Mercelis, P.; Van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2005, 11, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Wall, C.; Pham, H.; Esker, A.; Williams, C. Characterizing Binder–Powder Interaction in Binder Jetting Additive Manufacturing Via Sessile Drop Goniometry. J. Manuf. Sci. Eng. 2018, 141, 011005. [Google Scholar] [CrossRef]
- González, G.; Baruffaldi, D.; Martinengo, C.; Angelini, A.; Chiappone, A.; Roppolo, I.; Pirri, C.; Frascella, F. Materials Testing for the Development of Biocompatible Devices through Vat-Polymerization 3D Printing. Nanomaterials 2020, 10, 1788. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Zeng, K.; Pal, D.; Stucker, B.; A Review of Thermal Analysis Methods in Laser Sintering and Selective Laser Melting. 23rd Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, SFF 2012. Available online: http://utw10945.utweb.utexas.edu/Manuscripts/2012/2012-60-Zeng.pdf (accessed on 26 July 2021).
- Allahham, N.; Fina, F.; Marcuta, C.; Kraschew, L.; Mohr, W.; Gaisford, S.; Basit, A.W.; Goyanes, A. Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron. Pharmaceutics 2020, 12, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef]
- Leong, K.F.; Chua, C.K.; Gui, W.S. Verani Building Porous Biopolymeric Microstructures for Controlled Drug Delivery Devices Using Selective Laser Sintering. Int. J. Adv. Manuf. Technol. 2006, 31, 483–489. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Klauss, P.; Kanis, L.A. Laser Printing of PCL/Progesterone Tablets for Drug Delivery Applications in Hormone Cancer Therapy. Lasers Manuf. Mater. Process. 2017, 4, 108–120. [Google Scholar] [CrossRef]
- Salmoria, G.; Klauss, P.; Zepon, K.M.; Kanis, L.; Roesler, C.; Vieira, L. Development of functionally-graded reservoir of PCL/PG by selective laser sintering for drug delivery devices. Virtual Phys. Prototyp. 2012, 7, 107–115. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Trenfield, S.J.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology. Pharmaceutics 2019, 11, 148. [Google Scholar] [CrossRef] [Green Version]
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int. J. Pharm. 2018, 547, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Salmoria, G.V.; Vieira, F.E.; Ghizoni, G.B.; Gindri, I.M.; Kanis, L.A. Additive Manufacturing of PE/Fluorouracil Waffles for Implantable Drug Delivery in Bone Cancer Treatment. Int. J. Eng. Res. Sci. 2017, 3, 62–70. [Google Scholar] [CrossRef]
- Salmoria, G.; Cardenuto, M.; Roesler, C.; Zepon, K.; Kanis, L. PCL/Ibuprofen Implants Fabricated by Selective Laser Sintering for Orbital Repair. Procedia CIRP 2016, 49, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Gv, S.; Fe, V.; Gb, G.; Ms, M.; La, K. 3D printing of PCL/Fluorouracil tablets by selective laser sintering: Properties of implantable drug delivery for cartilage cancer treatment. Rheumatol. Orthop. Med. 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Salmoria, G.; Vieira, F.; Muenz, E.; Gindri, I.; Marques, M.; Kanis, L. Additive Manufacturing of PE/fluorouracil/progesterone intrauterine device for endometrial and ovarian cancer treatments. Polym. Test. 2018, 71, 312–317. [Google Scholar] [CrossRef]
- Davis, D.A.; Thakkar, R.; Su, Y.; Williams, R.O.; Maniruzzaman, M. Selective Laser Sintering 3-Dimensional Printing as a Single Step Process to Prepare Amorphous Solid Dispersion Dosage Forms for Improved Solubility and Dissolution Rate. J. Pharm. Sci. 2021, 110, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Abramov, Y.A.; Sun, G.; Zeng, Q.; Zeng, Q.; Yang, M. Guiding Lead Optimization for Solubility Improvement with Physics-Based Modeling. Mol. Pharm. 2020, 17, 666–673. [Google Scholar] [CrossRef]
- Lipp, R. The innovator pipeline: Bioavailability challenges and advanced oral drug delivery opportunities. Am. Pharm. Rev. 2013, 16. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/135982-The-Innovator-Pipeline-Bioavailability-Challenges-and-Advanced-Oral-Drug-Delivery-Opportunities/ (accessed on 26 July 2021).
- Brouwers, J.; Brewster, M.E.; Augustijns, P. Supersaturating Drug Delivery Systems: The Answer to Solubility-Limited Oral Bioavailability? J. Pharm. Sci. 2009, 98, 2549–2572. [Google Scholar] [CrossRef]
- Augustijns, P.; Brewster, M.E. Supersaturating Drug Delivery Systems: Fast is Not Necessarily Good Enough. J. Pharm. Sci. 2012, 101, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous solid dispersions: An update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int. J. Pharm. 2020, 586, 119560. [Google Scholar] [CrossRef]
- Wyttenbach, N.; Kuentz, M. Glass-forming ability of compounds in marketed amorphous drug products. Eur. J. Pharm. Biopharm. 2017, 112, 204–208. [Google Scholar] [CrossRef]
- DiNunzio, J.C.; Brough, C.; Miller, D.A.; Williams, R.O.; McGINITY, J.W. Applications of KinetiSol® Dispersing for the production of plasticizer free amorphous solid dispersions. Eur. J. Pharm. Sci. 2010, 40, 179–187. [Google Scholar] [CrossRef]
- Jermain, S.V.; Lowinger, M.B.; Ellenberger, D.J.; Miller, D.A.; Su, Y.; Williams, I.R.O.; Iii, R.W. In Vitro and In Vivo Behaviors of KinetiSol and Spray-Dried Amorphous Solid Dispersions of a Weakly Basic Drug and Ionic Polymer. Mol. Pharm. 2020, 17, 2789–2808. [Google Scholar] [CrossRef] [PubMed]
- Jermain, S.V.; Miller, D.; Spangenberg, A.; Lu, X.; Moon, C.; Su, Y.; Williams, R.O. Homogeneity of amorphous solid dispersions—An example with KinetiSol®. Drug Dev. Ind. Pharm. 2019, 45, 724–735. [Google Scholar] [CrossRef]
- DiNunzio, J.C.; Brough, C.; Hughey, J.R.; Miller, D.A.; Iii, R.W.; McGINITY, J.W. Fusion production of solid dispersions containing a heat-sensitive active ingredient by hot melt extrusion and Kinetisol® dispersing. Eur. J. Pharm. Biopharm. 2010, 74, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Gala, U.; Miller, D.; Iii, R.O.W. Improved Dissolution and Pharmacokinetics of Abiraterone through KinetiSol® Enabled Amorphous Solid Dispersions. Pharmaceutics 2020, 12, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughey, J.R.; DiNunzio, J.C.; Bennett, R.C.; Brough, C.; Miller, D.A.; Ma, H.; Iii, R.W.; McGINITY, J.W. Dissolution Enhancement of a Drug Exhibiting Thermal and Acidic Decomposition Characteristics by Fusion Processing: A Comparative Study of Hot Melt Extrusion and KinetiSol® Dispersing. AAPS Pharm. Sci. Tech. 2010, 11, 760–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakkar, R.; Thakkar, R.; Pillai, A.; Ashour, E.A.; Repka, M.A. Systematic screening of pharmaceutical polymers for hot melt extrusion processing: A comprehensive review. Int. J. Pharm. 2020, 576, 118989. [Google Scholar] [CrossRef]
- Thakkar, R.; Davis, D.A.; Williams, R.O.; Maniruzzaman, M. Selective Laser Sintering of a Photosensitive Drug: Impact of Processing and Formulation Parameters on Degradation, Solid-State, and Quality of 3D Printed Dosage Forms. bioRxiv 2021. [Google Scholar] [CrossRef]
- Thakkar, R.; Zhang, Y.; Zhang, J.; Maniruzzaman, M. Synergistic application of twin-screw granulation and selective laser sintering 3D printing for the development of pharmaceutical dosage forms with enhanced dissolution rates and physical properties. Eur. J. Pharm. Biopharm. 2021, 163, 141–156. [Google Scholar] [CrossRef]
- Thakkar, R.; Pillai, A.R.; Zhang, J.; Zhang, Y.; Kulkarni, V.; Maniruzzaman, M. Novel On-Demand 3-Dimensional (3-D) Printed Tablets Using Fill Density as an Effective Release-Controlling Tool. Polymers 2020, 12, 1872. [Google Scholar] [CrossRef]
- Sun, D.D.; Wen, H.; Taylor, L.S. Non-Sink Dissolution Conditions for Predicting Product Quality and In Vivo Performance of Supersaturating Drug Delivery Systems. J. Pharm. Sci. 2016, 105, 2477–2488. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.F.B.; Mohamed, E.; Ozkan, T.; Kuttolamadom, M.; Khan, M.A.; Asadi, A.; Rahman, Z. Understanding the effects of formulation and process variables on the printlets quality manufactured by selective laser sintering 3D printing. Int. J. Pharm. 2019, 570, 118651. [Google Scholar] [CrossRef]
- Jara, M.; Warnken, Z.; Williams, R. Amorphous Solid Dispersions and the Contribution of Nanoparticles to In Vitro Dissolution and In Vivo Testing: Niclosamide as a Case Study. Pharmaceutics 2021, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Ilevbare, G.A.; Taylor, L.S. Liquid–Liquid Phase Separation in Highly Supersaturated Aqueous Solutions of Poorly Water-Soluble Drugs: Implications for Solubility Enhancing Formulations. Cryst. Growth Des. 2013, 13, 1497–1509. [Google Scholar] [CrossRef]
- Luebbert, C.; Sadowski, G. Moisture-induced phase separation and recrystallization in amorphous solid dispersions. Int. J. Pharm. 2017, 532, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Blaabjerg, L.I.; Lindenberg, E.; Löbmann, K.; Grohganz, H.; Rades, T. Is there a correlation between the glass forming ability of a drug and its supersaturation propensity? Int. J. Pharm. 2018, 538, 243–249. [Google Scholar] [CrossRef]
- Blaabjerg, L.I.; Bulduk, B.; Lindenberg, E.; Löbmann, K.; Rades, T.; Grohganz, H. Influence of Glass Forming Ability on the Physical Stability of Supersaturated Amorphous Solid Dispersions. J. Pharm. Sci. 2019, 108, 2561–2569. [Google Scholar] [CrossRef]
- Baird, J.A.; Van Eerdenbrugh, B.; Taylor, L. A Classification System to Assess the Crystallization Tendency of Organic Molecules from Undercooled Melts. J. Pharm. Sci. 2010, 99, 3787–3806. [Google Scholar] [CrossRef]
- Moseson, D.E.; Corum, I.D.; Lust, A.; Altman, K.J.; Hiew, T.N.; Eren, A.; Nagy, Z.K.; Taylor, L.S. Amorphous Solid Dispersions Containing Residual Crystallinity: Competition between Dissolution and Matrix Crystallization. AAPS J. 2021, 23, 69. [Google Scholar] [CrossRef]
- Brika, S.E.; Letenneur, M.; Dion, C.A.; Brailovski, V. Influence of particle morphology and size distribution on the powder flowability and laser powder bed fusion manufacturability of Ti-6Al-4V alloy. Addit. Manuf. 2020, 31, 100929. [Google Scholar] [CrossRef]
- Hempel, N.; Knopp, M.M.; Berthelsen, R.; Zeitler, J.A.; Löbmann, K. The influence of drug and polymer particle size on the in situ amorphization using microwave irradiation. Eur. J. Pharm. Biopharm. 2020, 149, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Blaabjerg, L.I.; Lindenberg, E.; Löbmann, K.; Grohganz, H.; Rades, T. Glass Forming Ability of Amorphous Drugs Investigated by Continuous Cooling and Isothermal Transformation. Mol. Pharm. 2016, 13, 3318–3325. [Google Scholar] [CrossRef]










| Tablet Batch No. | Height (mm) | Diameter (mm) | Weight (mg) | Volume (mm3) | Density (kg/m3) | Hardness (kp) | Disintegration Time (s) |
|---|---|---|---|---|---|---|---|
| 50 mm/s PFS | 6.57 ± 0.06 | 12.90 ± 0.26 | 471 ± 4.04 | 857.82 | 0.549 | 8.66 ± 0.24 | 67 ± 5 |
| 75 mm/s PFS | 6.43 ± 0.06 | 12.77 ± 0.12 | 367 ± 8.74 | 823.12 | 0.446 | 6.35 ± 0.17 | 45 ± 12 |
| 100 mm/s PFS | 6.37 ± 0.06 | 12.97 ± 0.15 | 306 ± 6.56 | 840.31 | 0.364 | 4.56 ± 0.12 | 25 ± 9 |
| 50 mm/s UFS | 6.53 ± 0.15 | 12.87 ± 0.15 | 457 ± 10.41 | 849.06 | 0.538 | 7.52 ± 0.39 | 58 ± 14 |
| 75 mm/s UFS | 6.43 ± 0.06 | 12.53 ± 0.47 | 346 ± 6.56 | 793.30 | 0.436 | 6.03 ± 0.19 | 36 ± 8 |
| 100 mm/s UFS | 6.23 ± 0.06 | 12.80 ± 0.10 | 287 ± 6.56 | 801.70 | 0.358 | 4.00 ± 0.33 | 7 ± 3 |
| Sample | Mean Particle Size | PDI |
|---|---|---|
| Unfiltered 100 mm/s UFS | 206.20 ± 9.95 | 0.399 ± 0.087 |
| Filtered 100 mm/s UFS | 9.87 ± 0.03 | 0.219 ± 0.001 |
| Unfiltered 100 mm/s PFS | 200.83 ± 1.12 | 0.272 ± 0.030 |
| Filtered 100 mm/s PFS | 9.61 ± 0.02 | 0.200 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakkar, R.; Jara, M.O.; Swinnea, S.; Pillai, A.R.; Maniruzzaman, M. Impact of Laser Speed and Drug Particle Size on Selective Laser Sintering 3D Printing of Amorphous Solid Dispersions. Pharmaceutics 2021, 13, 1149. https://doi.org/10.3390/pharmaceutics13081149
Thakkar R, Jara MO, Swinnea S, Pillai AR, Maniruzzaman M. Impact of Laser Speed and Drug Particle Size on Selective Laser Sintering 3D Printing of Amorphous Solid Dispersions. Pharmaceutics. 2021; 13(8):1149. https://doi.org/10.3390/pharmaceutics13081149
Chicago/Turabian StyleThakkar, Rishi, Miguel O. Jara, Steve Swinnea, Amit R. Pillai, and Mohammed Maniruzzaman. 2021. "Impact of Laser Speed and Drug Particle Size on Selective Laser Sintering 3D Printing of Amorphous Solid Dispersions" Pharmaceutics 13, no. 8: 1149. https://doi.org/10.3390/pharmaceutics13081149
APA StyleThakkar, R., Jara, M. O., Swinnea, S., Pillai, A. R., & Maniruzzaman, M. (2021). Impact of Laser Speed and Drug Particle Size on Selective Laser Sintering 3D Printing of Amorphous Solid Dispersions. Pharmaceutics, 13(8), 1149. https://doi.org/10.3390/pharmaceutics13081149