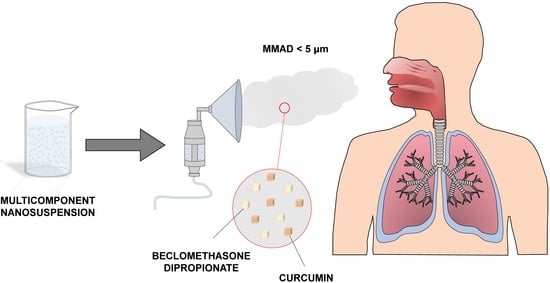
Pulmonary Delivery of Curcumin and Beclomethasone Dipropionate in a Multicomponent Nanosuspension for the Treatment of Bronchial Asthma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanosuspension
2.3. Particle Size Analysis
2.4. Scanning Electron Microscopy
2.5. Solubility Studies
2.6. Solid State Characterization
2.7. Preparation of Nanosuspension
2.8. Nebulization and Aerodynamic Behaviour of Nanosuspensions
2.9. HPLC Analysis
2.10. Statistical Analysis of Data
3. Results and Discussion
3.1. Preparation and Characterization of Nanosuspension
3.2. DSC Analysis
3.3. ATR-FTIR Analysis
3.4. XRPD Analysis
3.5. Preparation of the Multicomponent Nanosuspension
3.6. Nebulization Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carr, T.F.; Bleecker, E. Asthma heterogeneity and severity. World Allergy Organ. J. 2016, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Nakagome, K.; Nagata, M. Pathogenesis of airway inflammation in bronchial asthma. Auris Nasus Larynx 2011, 38, 555–563. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Christman, J.W.; Sadikot, R.T.; Blackwell, T.S. The Role of Nuclear Factor-κ B in Pulmonary Diseases. Chest 2000, 117, 1482–1487. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention; Global Initiative for Asthma: Fontana, WI, USA, 2020. [Google Scholar]
- Park, H.S.; Kim, S.R.; Kim, J.O.; Lee, Y.C. The Roles of Phytochemicals in Bronchial Asthma. Molecules 2010, 15, 6810–6834. [Google Scholar] [CrossRef]
- Abidi, A.; Gupta, S.; Agarwal, M.; Bhalla, H.; Saluja, M. Evaluation of Efficacy of Curcumin as an Add-on therapy in Patients of Bronchial Asthma. J. Clin. Diagn. Res. 2014, 8, HC19–HC24. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-W.; Cha, J.-Y.; Jung, J.-E.; Chang, B.-C.; Kwon, H.-J.; Lee, B.-R.; Kim, D.-Y. Curcumin attenuates allergic airway inflammation and hyper-responsiveness in mice through NF-κB inhibition. J. Ethnopharmacol. 2011, 136, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.; Zhang, W.; Nie, Y.; Yu, G.; Liu, L.; Lin, L.; Wen, S.; Zhu, L.; Li, C. Protective Effect of Curcumin on Acute Airway Inflammation of Allergic Asthma in Mice Through Notch1–GATA3 Signaling Pathway. Inflammation 2014, 37, 1476–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahid, H.; Shahzad, M.; Shabbir, A.; Saghir, G. Immunomodulatory and Anti-Inflammatory Potential of Curcumin for the Treatment of Allergic Asthma: Effects on Expression Levels of Pro-inflammatory Cytokines and Aquaporins. Inflammation 2019, 42, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Schlich, M.; Pireddu, R.; Fadda, A.M.; Sinico, C. Nanocrystals as Effective Delivery Systems of Poorly Water-soluble Natural Molecules. Curr. Med. Chem. 2019, 26, 4657–4680. [Google Scholar] [CrossRef]
- Lai, F.; Schlich, M.; Pireddu, R.; Corrias, F.; Fadda, A.; Sinico, C. Production of nanosuspensions as a tool to improve drug bioavailability: Focus on topical delivery. Curr. Pharm. Des. 2015, 21, 6089–6103. [Google Scholar] [CrossRef]
- Mosharraf, M.; Nyström, C. The effect of particle size and shape on the surface specific dissolution rate of microsized practically insoluble drugs. Int. J. Pharm. 1995, 122, 35–47. [Google Scholar] [CrossRef]
- Müller, R.H.; Peters, K. Nanosuspensions for the formulation of poorly soluble drugs: I. Preparation by a size-reduction technique. Int. J. Pharm. 1998, 160, 229–237. [Google Scholar] [CrossRef]
- Jacobs, C.; Müller, R.H. Production and Characterization of a Budesonide Nanosuspension for Pulmonary Administration. Pharm. Res. 2002, 19, 189–194. [Google Scholar] [CrossRef]
- Chiang, P.-C.; Alsup, J.W.; Lai, Y.; Hu, Y.; Heyde, B.R.; Tung, D. Evaluation of Aerosol Delivery of Nanosuspension for Pre-clinical Pulmonary Drug Delivery. Nanoscale Res. Lett. 2009, 4, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, J. Preparation of budesonide nanosuspensions for pulmonary delivery: Characterization, in vitro release and in vivo lung distribution studies. Artif. Cells Nanomed. Biotechnol. 2016, 44, 285–289. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Van den Mooter, G.; Augustijns, P. Top-down production of drug nanocrystals: Nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008, 364, 64–75. [Google Scholar] [CrossRef]
- Britland, S.; Finter, W.; Chrystyn, H.; Eagland, D.; Abdelrahim, M.E. Droplet aerodynamics, cellular uptake, and efficacy of a nebulizable corticosteroid nanosuspension are superior to a micronized dosage form. Biotechnol. Prog. 2012, 28, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Z.; Young, A.L.; Chiang, P.-C.; Thurston, A.; Pretzer, D.K. Fluticasone and budesonide nanosuspensions for pulmonary delivery: Preparation, characterization, and pharmacokinetic studies. J. Pharm. Sci. 2008, 97, 4869–4878. [Google Scholar] [CrossRef]
- Dompeling, E.; Van Schayck, C.P.; Molema, J.; Folgering, H.; Van Grunsven, P.M.; Van Weel, C. Inhaled beclomethasone improves the course of asthma and COPD. Eur. Respir. J 1992, 5, 945–952. [Google Scholar] [PubMed]
- Corrias, F.; Schlich, M.; Sinico, C.; Pireddu, R.; Valenti, D.; Fadda, A.M.; Marceddu, S.; Lai, F. Nile red nanosuspensions as investigative model to study the follicular targeting of drug nanocrystals. Int. J. Pharm. 2017, 524, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Casula, L.; Sinico, C.; Valenti, D.; Pini, E.; Pireddu, R.; Schlich, M.; Lai, F.; Fadda, A.M. Delivery of beclomethasone dipropionate nanosuspensions with an electronic cigarette. Int. J. Pharm. 2021, 596, 120293. [Google Scholar] [CrossRef]
- Berg, E.; Lamb, P.; Ali, A.; Dennis, J.; Tservistas, M.; Mitchell, J. Assessment of the need to coat particle collection cups of the NGI to mitigate droplet bounce when evaluating nebuliser-produced droplets. Pharmeuropa Sci. Notes 2008, 2008, 21–25. [Google Scholar]
- Marple, V.A.; Olson, B.A.; Santhanakrishnan, K.; Roberts, D.L.; Mitchell, J.P.; Hudson-Curtis, B.L. Next Generation Pharmaceutical Impactor: A New Impactor for Pharmaceutical Inhaler Testing. Part III. Extension of Archival Calibration to 15 L/min. J. Aerosol Med. 2004, 17, 335–343. [Google Scholar] [CrossRef]
- Manca, M.L.; Peris, J.E.; Melis, V.; Valenti, D.; Cardia, M.C.; Lattuada, D.; Escribano-Ferrer, E.; Fadda, A.M.; Manconi, M. Nanoincorporation of curcumin in polymer-glycerosomes and evaluation of their in vitro–in vivo suitability as pulmonary delivery systems. RSC Adv. 2015, 5, 105149–105159. [Google Scholar] [CrossRef]
- Li, X.; Yuan, H.; Zhang, C.; Chen, W.; Cheng, W.; Chen, X.; Ye, X. Preparation and in-vitro/in-vivo evaluation of curcumin nanosuspension with solubility enhancement. J. Pharm. Pharmacol. 2016, 68, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, H.; Komasaka, T.; Tomari, T.; Kitano, Y.; Takekawa, K. Nanosuspension formulations of poorly water-soluble compounds for intravenous administration in exploratory toxicity studies:in vitroandin vivoevaluation. J. Appl. Toxicol. 2016, 36, 1259–1267. [Google Scholar] [CrossRef]
- Wang, L.; Du, J.; Zhou, Y.; Wang, Y. Safety of nanosuspensions in drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 455–469. [Google Scholar] [CrossRef]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [Green Version]
- Zani, F.; Veneziani, C.; Bazzoni, E.; Maggi, L.; Caponetti, G.; Bettini, R. Sterilization of corticosteroids for ocular and pulmonary delivery with supercritical carbon dioxide. Int. J. Pharm. 2013, 450, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.J.; Younis, U.S.; Myrdal, P.B. Estimating the Aqueous Solubility of Pharmaceutical Hydrates. J. Pharm. Sci. 2016, 105, 1914–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Peters, J.I.; Williams, R.O., III. Inhaled nanoparticles—A current review. Int. J. Pharm. 2008, 356, 239–247. [Google Scholar] [CrossRef] [PubMed]






| Formulations | Composition | Dimensional Analysis | |||
|---|---|---|---|---|---|
| Curcumin (% w/w) | Beclomethasone Dipropionate (% w/w) | P188 (% w/w) | Average Diameter (nm) | PDI | |
| CUR-NS | 1 | - | 0.5 | 202 ± 5 | 0.23 ± 0.02 |
| BDP-NS | - | 1 | 0.5 | 241 ± 2 | 0.24 ± 0.01 |
| CUR+BDP-NS | 0.5 | 0.5 | 0.5 | 221 ± 7 | 0.25 ± 0.02 |
| Aerodynamic Parameters | CUR+BDP-NS | |||
|---|---|---|---|---|
| CUR-NS | CUR | BDP | BDP-NS | |
| ED% | 57.0 ± 0.9 | 81.9 ± 1.1 | 83.4 ± 3.7 | 65.5 ± 4.9 |
| FPD (mg) | 7.8 ± 0.3 | 6.8 ± 0.8 | 6.1 ± 0.1 | 7.6 ± 0.2 |
| FPF (%) | 60.3 ± 1.9 § | 64.7 ± 4.0 § | 62.7 ± 0.5 § | 68.1 ± 7.2 § |
| MMAD (µm) | 4.1 ± 0.1 | 3.4 ± 0.6 | 3.8 ± 0.1 | 3.7 ± 0.2 |
| GSD | 2.6 ± 0.1 | 3.1 ± 0.4 | 2.9 ± 0.1 | 2.6 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casula, L.; Lai, F.; Pini, E.; Valenti, D.; Sinico, C.; Cardia, M.C.; Marceddu, S.; Ailuno, G.; Fadda, A.M. Pulmonary Delivery of Curcumin and Beclomethasone Dipropionate in a Multicomponent Nanosuspension for the Treatment of Bronchial Asthma. Pharmaceutics 2021, 13, 1300. https://doi.org/10.3390/pharmaceutics13081300
Casula L, Lai F, Pini E, Valenti D, Sinico C, Cardia MC, Marceddu S, Ailuno G, Fadda AM. Pulmonary Delivery of Curcumin and Beclomethasone Dipropionate in a Multicomponent Nanosuspension for the Treatment of Bronchial Asthma. Pharmaceutics. 2021; 13(8):1300. https://doi.org/10.3390/pharmaceutics13081300
Chicago/Turabian StyleCasula, Luca, Francesco Lai, Elena Pini, Donatella Valenti, Chiara Sinico, Maria Cristina Cardia, Salvatore Marceddu, Giorgia Ailuno, and Anna Maria Fadda. 2021. "Pulmonary Delivery of Curcumin and Beclomethasone Dipropionate in a Multicomponent Nanosuspension for the Treatment of Bronchial Asthma" Pharmaceutics 13, no. 8: 1300. https://doi.org/10.3390/pharmaceutics13081300
APA StyleCasula, L., Lai, F., Pini, E., Valenti, D., Sinico, C., Cardia, M. C., Marceddu, S., Ailuno, G., & Fadda, A. M. (2021). Pulmonary Delivery of Curcumin and Beclomethasone Dipropionate in a Multicomponent Nanosuspension for the Treatment of Bronchial Asthma. Pharmaceutics, 13(8), 1300. https://doi.org/10.3390/pharmaceutics13081300