Amphiphilic Poly(N-vinylpyrrolidone) Nanoparticles Conjugated with DR5-Specific Antitumor Cytokine DR5-B for Targeted Delivery to Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Reagents and Cell Lines
2.2. Expression and Purification of Recombinant Protein DR5-B
2.3. Production of P-DR5-B Nanoparticles
2.3.1. Synthesis of Amph-PVP-Cl (OD11000)
2.3.2. Potassium Maleimide Synthesis
2.3.3. Modification of Amph-PVP-Cl by Maleimide Group
2.3.4. Amph-PVP Nanoparticles Preparation
2.3.5. Conjugation of the Amph-PVP Nanoparticles with DR5-B
2.4. Imaging Nanoparticles by TEM and AFM
2.5. Cell Culture and Multicellular Tumor Spheroids Formation
2.6. Cytotoxicity Evaluation
2.7. Statistical Analysis
3. Results
3.1. Preparation of Amph-PVP Nanoparticles
3.2. Conjugation of the Amph-PVP Nanoparticles with the DR5-B Protein
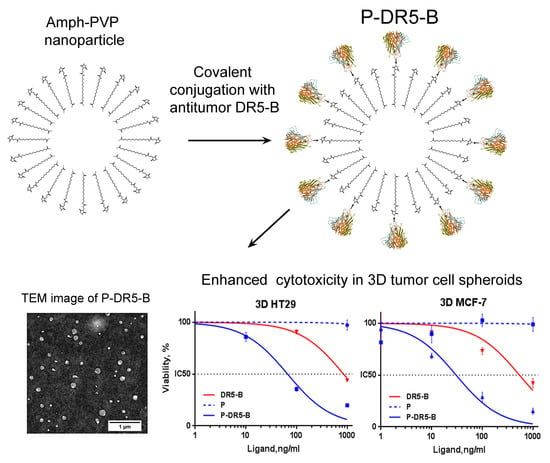
3.3. Cytotoxicity Study of the P-DR5-B Nanoparticles in 2D and 3D In Vitro Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Q.-Y.; Xu, Y.-M.; Lau, A.T.Y. Recent Progress of Nanocarrier-Based Therapy for Solid Malignancies. Cancers 2020, 12, 2783. [Google Scholar] [CrossRef] [PubMed]
- Thotakura, N.; Parashar, P.; Raza, K. Assessing the Pharmacokinetics and Toxicology of Polymeric Micelle Conjugated Therapeutics. Expert Opin. Drug Metab. Toxicol. 2021, 17, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Lai, T.C.; Tomoda, K.; Kwon, G.S. Polymeric Micelles for Multi-Drug Delivery in Cancer. AAPS PharmSciTech 2015, 16, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Lin, W.; Ma, G.; Kampf, N.; Yuan, Z.; Chen, S. Development of Long-Circulating Zwitterionic Cross-Linked Micelles for Active-Targeted Drug Delivery. Biomacromolecules 2016, 17, 2010–2018. [Google Scholar] [CrossRef]
- Miura, Y.; Takenaka, T.; Toh, K.; Wu, S.; Nishihara, H.; Kano, M.R.; Ino, Y.; Nomoto, T.; Matsumoto, Y.; Koyama, H.; et al. Cyclic RGD-Linked Polymeric Micelles for Targeted Delivery of Platinum Anticancer Drugs to Glioblastoma through the Blood–Brain Tumor Barrier. ACS Nano 2013, 7, 8583–8592. [Google Scholar] [CrossRef]
- Sabzi, A.; Rahmani, A.; Edalati, M.; Kahroba, H.; Dadpour, M.R.; Salehi, R.; Zarebkohan, A. Targeted Co-Delivery of Curcumin and Doxorubicin by Citric Acid Functionalized Poly (ε-Caprolactone) Based Micelle in MDA-MB-231 Cell. Colloids Surf. B 2020, 194, 111225. [Google Scholar] [CrossRef]
- Waleka, E.; Stojek, Z.; Karbarz, M. Activity of Povidone in Recent Biomedical Applications with Emphasis on Micro- and Nano Drug Delivery Systems. Pharmaceutics 2021, 13, 654. [Google Scholar] [CrossRef]
- Kuskov, A.N.; Kulikov, P.P.; Goryachaya, A.V.; Tzatzarakis, M.N.; Docea, A.O.; Velonia, K.; Shtilman, M.I.; Tsatsakis, A.M. Amphiphilic Poly-N-Vinylpyrrolidone Nanoparticles as Carriers for Non-Steroidal, Anti-Inflammatory Drugs: In Vitro Cytotoxicity and in Vivo Acute Toxicity Study. Nanomedicine 2017, 13, 1021–1030. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Stratidakis, A.K.; Goryachaya, A.V.; Tzatzarakis, M.N.; Stivaktakis, P.D.; Docea, A.O.; Berdiaki, A.; Nikitovic, D.; Velonia, K.; Shtilman, M.I.; et al. In Vitro Blood Compatibility and in Vitro Cytotoxicity of Amphiphilic Poly-N-Vinylpyrrolidone Nanoparticles. Food Chem. Toxicol. 2019, 127, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Villemson, A.L.; Kuskov, A.N.; Shtilman, M.I.; Galebskaya, L.V.; Ryumina, E.V.; Larionova, N.I. Interaction of Polymer Aggregates Based on Stearoyl-Poly-N-Vinylpyrrolidone with Blood Components. Biochemistry 2004, 69, 621–628. [Google Scholar] [CrossRef]
- Yamskov, I.A.; Kuskov, A.N.; Babievsky, K.K.; Berezin, B.B.; Krayukhina, M.A.; Samoylova, N.A.; Tikhonov, V.E.; Shtilman, M.I. Novel Liposomal Forms of Antifungal Antibiotics Modified by Amphiphilic Polymers. Appl. Biochem. Microbiol. 2008, 44, 624–628. [Google Scholar] [CrossRef]
- Kuskov, A.N.; Villemson, A.L.; Shtilman, M.I.; Larionova, N.I.; Tsatsakis, A.M.; Tsikalas, I.; Rizos, A.K. Amphiphilic Poly-N-Vinylpyrrolidone Nanocarriers with Incorporated Model Proteins. J. Phys. Condens. Matter. 2007, 19, 205139. [Google Scholar] [CrossRef]
- Kuskov, A.N.; Shtilman, M.I.; Goryachaya, A.V.; Tashmuhamedov, R.I.; Yaroslavov, A.A.; Torchilin, V.P.; Tsatsakis, A.M.; Rizos, A.K. Self-Assembling Nanoscaled Drug Delivery Systems Composed of Amphiphilic Poly-N-Vinylpyrrolidones. J. Non. Cryst. Solids 2007, 353, 3969–3975. [Google Scholar] [CrossRef]
- Basyreva, L.Y.; Voinova, E.V.; Gusev, A.A.; Mikhalchik, E.V.; Kuskov, A.N.; Goryachaya, A.V.; Gusev, S.A.; Shtilman, M.I.; Velonia, K.; Tsatsakis, A.M. Fluorouracil Neutrophil Extracellular Traps Formation Inhibited by Polymer Nanoparticle Shielding. Mater. Sci. Eng. 2020, 108, 110382. [Google Scholar] [CrossRef] [PubMed]
- Berdiaki, A.; Perisynaki, E.; Stratidakis, A.; Kulikov, P.P.; Kuskov, A.N.; Stivaktakis, P.; Henrich-Noack, P.; Luss, A.L.; Shtilman, M.M.; Tzanakakis, G.N.; et al. Assessment of Amphiphilic Poly-N-Vinylpyrrolidone Nanoparticles’ Biocompatibility with Endothelial Cells in Vitro and Delivery of an Anti-Inflammatory Drug. Mol. Pharm. 2020, 17, 4212–4225. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Pai, R.C.; Fong, S.; Leung, S.; Lawrence, D.A.; Marsters, S.A.; Blackie, C.; Chang, L.; McMurtrey, A.E.; Hebert, A.; et al. Safety and Antitumor Activity of Recombinant Soluble Apo2 Ligand. J. Clin. Investig. 1999, 104, 155–162. [Google Scholar] [CrossRef]
- Ouyang, X.; Shi, M.; Jie, F.; Bai, Y.; Shen, P.; Yu, Z.; Wang, X.; Huang, C.; Tao, M.; Wang, Z.; et al. Phase III Study of Dulanermin (Recombinant Human Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand/Apo2 Ligand) Combined with Vinorelbine and Cisplatin in Patients with Advanced Non-Small-Cell Lung Cancer. Investig. New Drugs 2018, 36, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Kelley, S.K.; Harris, L.A.; Xie, D.; DeForge, L.; Totpal, K.; Bussiere, J.; Fox, J.A. Preclinical Studies to Predict the Disposition of Apo2L/Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand in Humans: Characterization of in Vivo Efficacy, Pharmacokinetics, and Safety. J. Pharmacol. Exp. Ther. 2001, 299, 31. [Google Scholar]
- De Miguel, D.; Lemke, J.; Anel, A.; Walczak, H.; Martinez-Lostao, L. Onto Better TRAILs for Cancer Treatment. Cell Death Differ. 2016, 23, 733–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparian, M.E.; Chernyak, B.V.; Dolgikh, D.A.; Yagolovich, A.V.; Popova, E.N.; Sycheva, A.M.; Moshkovskii, S.A.; Kirpichnikov, M.P. Generation of New TRAIL Mutants DR5-A and DR5-B with Improved Selectivity to Death Receptor 5. Apoptosis 2009, 14, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Yagolovich, A.V.; Artykov, A.A.; Karmakova, T.A.; Vorontsova, M.S.; Pankratov, A.A.; Andreev-Andrievsky, A.A.; Dolgikh, D.A.; Kirpichnikov, M.P.; Gasparian, M.E. Genetically Modified DR5-Specific TRAIL Variant DR5-B Revealed Dual Antitumor and Protumoral Effect in Colon Cancer Xenografts and an Improved Pharmacokinetic Profile. Transl. Oncol. 2020, 13, 100762. [Google Scholar] [CrossRef]
- Belkahla, H.; Herlem, G.; Picaud, F.; Gharbi, T.; Hémadi, M.; Ammar, S.; Micheau, O. TRAIL–NP Hybrids for Cancer Therapy: A Review. Nanoscale 2017, 9, 5755–5768. [Google Scholar] [CrossRef] [PubMed]
- Yagolovich, A.V.; Artykov, A.A.; Dolgikh, D.A.; Kirpichnikov, M.P.; Gasparian, M.E. A New Efficient Method for Production of Recombinant Antitumor Cytokine TRAIL and Its Receptor-Selective Variant DR5-B. Biochemistry 2019, 84, 627–636. [Google Scholar] [CrossRef]
- Kuskov, A.N.; Kulikov, P.P.; Luss, A.L.; Goryachaya, A.V.; Shtil’man, M.I. Preparation of Polymer Nanoparticles by Self-Assembling of Amphiphilic Poly-N-Vinylpyrrolidone Derivatives in Aqueous Media. Russ. J. Appl. Chem. 2016, 89, 1461–1468. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nečas, D.; Klapetek, P. Gwyddion: An Open-Source Software for SPM Data Analysis. Open Phys. J. 2012, 10, s11534–s011. [Google Scholar] [CrossRef]
- Akasov, R.; Zaytseva-Zotova, D.; Burov, S.; Leko, M.; Dontenwill, M.; Chiper, M.; Vandamme, T.; Markvicheva, E. Formation of Multicellular Tumor Spheroids Induced by Cyclic RGD-Peptides and Use for Anticancer Drug Testing in Vitro. Int. J. Pharm. 2016, 506, 148–157. [Google Scholar] [CrossRef]
- Kulikov, P.P.; Kuskov, A.N.; Goryachaya, A.V.; Luss, A.N.; Shtil’man, M.I. Amphiphilic Poly-n-Vinyl-2-Pyrrolidone: Synthesis, Properties, Nanoparticles. Polym. Sci. Ser. D 2017, 10, 263–268. [Google Scholar] [CrossRef]
- Sadeghi, R.; Etemad, S.G.; Keshavarzi, E.; Haghshenasfard, M. Investigation of Alumina Nanofluid Stability by UV–Vis Spectrum. Microfluid Nanofluid 2015, 18, 1023–1030. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Millard, M.; Yakavets, I.; Zorin, V.; Kulmukhamedova, A.; Marchal, S.; Bezdetnaya, L. Drug Delivery to Solid Tumors: The Predictive Value of the Multicellular Tumor Spheroid Model for Nanomedicine Screening. IJN 2017, 12, 7993–8007. [Google Scholar] [CrossRef] [Green Version]
- Tawfik, M.; Hadlak, S.; Götze, C.; Sokolov, M.; Kulikov, P.; Kuskov, A.; Shtilman, M.; Sahel, B.A.; Henrich-Noack, P. Live In-Vivo Neuroimaging Reveals the Transport of Lipophilic Cargo Through the Blood-Retina Barrier with Modified Amphiphilic Poly-N-Vinylpyrrolidone Nanoparticles. J. Biomed. Nanotechnol. 2021, 17, 846–858. [Google Scholar] [CrossRef]
- Ng, W.; Yeong, W.; Naing, M. Polyvinylpyrrolidone-Based Bio-Ink Improves Cell Viability and Homogeneity during Drop-On-Demand Printing. Materials 2017, 10, 190. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. The Use of Poly(N-Vinyl Pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Moncalvo, F.; Martinez Espinoza, M.I.; Cellesi, F. Nanosized Delivery Systems for Therapeutic Proteins: Clinically Validated Technologies and Advanced Development Strategies. Front. Bioeng. Biotechnol. 2020, 8, 89. [Google Scholar] [CrossRef]
- Kaneda, Y.; Tsutsumi, Y.; Yoshioka, Y.; Kamada, H.; Yamamoto, Y.; Kodaira, H.; Tsunoda, S.; Okamoto, T.; Mukai, Y.; Shibata, H.; et al. The Use of PVP as a Polymeric Carrier to Improve the Plasma Half-Life of Drugs. Biomaterials 2004, 25, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Baganizi, D.; Nyairo, E.; Duncan, S.; Singh, S.; Dennis, V. Interleukin-10 Conjugation to Carboxylated PVP-Coated Silver Nanoparticles for Improved Stability and Therapeutic Efficacy. Nanomaterials 2017, 7, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuskov, A.N.; Voskresenskaya, A.A.; Goryachaya, A.V.; Artyukhov, A.A.; Shtilman, M.I.; Tsatsakis, A.M. Preparation and Characterization of Amphiphilic Poly-N-Vinylpyrrolidone Nanoparticles Containing Indomethacin. J. Mater. Sci. Mater. Med. 2010, 21, 1521–1530. [Google Scholar] [CrossRef]
- Kuskov, A.N.; Voskresenskaya, A.A.; Goryachaya, A.V.; Shtilman, M.I.; Spandidos, D.A.; Rizos, A.K.; Tsatsakis, A.M. Amphiphilic Poly-N-Vinylpyrrolidone Nanoparticles as Carriers for Non-Steroidal Anti-Inflammatory Drugs: Characterization and in Vitro Controlled Release of Indomethacin. Int. J. Mol. Med. 2010, 26, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Luss, A.L.; Kulikov, P.P.; Romme, S.B.; Andersen, C.L.; Pennisi, C.P.; Docea, A.O.; Kuskov, A.N.; Velonia, K.; Mezhuev, Y.O.; Shtilman, M.I.; et al. Nanosized Carriers Based on Amphiphilic Poly-N-Vinyl-2-Pyrrolidone for Intranuclear Drug Delivery. Nanomedicine 2018, 13, 703–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Lin, F.; You, D.; Qian, Y.; Wang, Y.; Bi, Y. Self-Assembly and Enzyme Responsiveness of Amphiphilic Linear-Dendritic Block Copolymers Based on Poly(N-Vinylpyrrolidone) and Dendritic Phenylalanyl-Lysine Dipeptides. Polymers 2019, 11, 1625. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.M.; Flores, H.; Gogineni, A.; Marsters, S.; Lawrence, D.A.; Kelley, R.F.; Ngu, H.; Sagolla, M.; Komuves, L.; Bourgon, R.; et al. Enhancing the Antitumor Efficacy of a Cell-Surface Death Ligand by Covalent Membrane Display. Proc. Natl. Acad. Sci. USA 2015, 112, 5679–5684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.L.Z.; Dhillon, S.H.K.; Wang, Y.; Pervaiz, S.; Fan, W.; Yang, Y.Y. Synergistic Anti-Cancer Effects via Co-Delivery of TNF-Related Apoptosis-Inducing Ligand (TRAIL/Apo2L) and Doxorubicin Using Micellar Nanoparticles. Mol. BioSyst. 2011, 7, 1512. [Google Scholar] [CrossRef] [PubMed]
- Skidan, I.; Miao, B.; Thekkedath, R.V.; Dholakia, P.; Degterev, A.; Torchilin, V. In Vitro Cytotoxicity of Novel Pro-Apoptotic Agent DM-PIT-1 in PEG-PE-Based Micelles Alone and in Combination with TRAIL. Drug Deliv. 2009, 16, 45–51. [Google Scholar] [CrossRef]
- Feng, C.; Han, X.; Chi, L.; Sun, J.; Gong, F.; Shen, Y. Synthesis, Characterization, and in Vitro Evaluation of TRAIL-Modified, Cabazitaxel -Loaded Polymeric Micelles for Achieving Synergistic Anticancer Therapy. J. Biomater. Sci. Polym. Ed. 2018, 29, 1729–1744. [Google Scholar] [CrossRef]
- Ajorlou, E.; Khosroushahi, A.Y.; Yeganeh, H. Novel Water-Borne Polyurethane Nanomicelles for Cancer Chemotherapy: Higher Efficiency of Folate Receptors Than TRAIL Receptors in a Cancerous Balb/C Mouse Model. Pharm. Res. 2016, 33, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Moyer, T.J.; Chen, F.; Toft, D.J.; Ruff, Y.; Cryns, V.L.; Stupp, S.I. Self-Assembled Peptide Nanostructures Targeting Death Receptor 5 and Encapsulating Paclitaxel As a Multifunctional Cancer Therapy. ACS Biomater. Sci. Eng. 2019, 5, 6046–6053. [Google Scholar] [CrossRef]
- Azijli, K.; Weyhenmeyer, B.; Peters, G.J.; de Jong, S.; Kruyt, F.A.E. Non-Canonical Kinase Signaling by the Death Ligand TRAIL in Cancer Cells: Discord in the Death Receptor Family. Cell Death Differ. 2013, 20, 858–868. [Google Scholar] [CrossRef] [Green Version]
- Prothionamide. Tuberculosis 2008, 88, 139–140. [CrossRef]
- Zanoni, M.; Pignatta, S.; Arienti, C.; Bonafè, M.; Tesei, A. Anticancer Drug Discovery Using Multicellular Tumor Spheroid Models. Expert Opin. Drug Discov. 2019, 14, 289–301. [Google Scholar] [CrossRef]
- Lazzari, G.; Couvreur, P.; Mura, S. Multicellular Tumor Spheroids: A Relevant 3D Model for the in Vitro Preclinical Investigation of Polymer Nanomedicines. Polym. Chem. 2017, 8, 4947–4969. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Stenzel, M.H. Multicellular Tumor Spheroids (MCTS) as a 3D In Vitro Evaluation Tool of Nanoparticles. Small 2018, 14, 1702858. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of Applying Multicellular Tumor Spheroids in Preclinical Phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef]
- Zakaria, A.B.; Picaud, F.; Rattier, T.; Pudlo, M.; Saviot, L.; Chassagnon, R.; Lherminier, J.; Gharbi, T.; Micheau, O.; Herlem, G. Nanovectorization of TRAIL with Single Wall Carbon Nanotubes Enhances Tumor Cell Killing. Nano Lett. 2015, 15, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Huet, H.A.; Growney, J.D.; Johnson, J.A.; Li, J.; Bilic, S.; Ostrom, L.; Zafari, M.; Kowal, C.; Yang, G.; Royo, A.; et al. Multivalent Nanobodies Targeting Death Receptor 5 Elicit Superior Tumor Cell Killing through Efficient Caspase Induction. mAbs 2014, 6, 1560–1570. [Google Scholar] [CrossRef]
- De Miguel, D.; Gallego-Lleyda, A.; Ayuso, J.M.; Pejenaute-Ochoa, D.; Jarauta, V.; Marzo, I.; Fernández, L.J.; Ochoa, I.; Conde, B.; Anel, A.; et al. High-Order TRAIL Oligomer Formation in TRAIL-Coated Lipid Nanoparticles Enhances DR5 Cross-Linking and Increases Antitumour Effect against Colon Cancer. Cancer Lett. 2016, 383, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Chekkat, N.; Lombardo, C.M.; Seguin, C.; Lechner, M.-C.; Dufour, F.; Nominé, Y.; De Giorgi, M.; Frisch, B.; Micheau, O.; Guichard, G.; et al. Relationship between the Agonist Activity of Synthetic Ligands of TRAIL-R2 and Their Cell Surface Binding Modes. Oncotarget 2018, 9, 15566–15578. [Google Scholar] [CrossRef] [Green Version]
- Naval, J.; de Miguel, D.; Gallego-Lleyda, A.; Anel, A.; Martinez-Lostao, L. Importance of TRAIL Molecular Anatomy in Receptor Oligomerization and Signaling. Implications for Cancer Therapy. Cancers 2019, 11, 444. [Google Scholar] [CrossRef] [Green Version]





| Samples | P | P-DR5-B | ||
|---|---|---|---|---|
| maleimide-modified to unmodified Amph-PVPs molar ratio | 1:3 | 1:1 | 1:3 | 1:1 |
| median size, nm 1 | 217.6 | 205.6 | 228.5 | 228.2 |
| polydispersity index 2 | 0.299 | 0.302 | 0.274 | 0.303 |
| amount of DR5-B, μg per 1 mg of the lyophilized nanoparticles | - | - | 4.0 ± 0.06 | 5.0 ± 0.05 |
| Ligand | 2D MCF-7 | 3D MCF-7 | 3D HCT116 | 3D HT29 | ||||
|---|---|---|---|---|---|---|---|---|
| Incubation Time, h | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 |
| DR5-B | 901.3 | 275.5 | >1000 | 584.0 | 16.3 | 5.0 | >1000 | 863.1 |
| P-DR5-B | 39.8 | 21.6 | 72.1 | 31.7 | 5.9 | 2.8 | 184.2 | 68.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagolovich, A.; Kuskov, A.; Kulikov, P.; Kurbanova, L.; Bagrov, D.; Artykov, A.; Gasparian, M.; Sizova, S.; Oleinikov, V.; Gileva, A.; et al. Amphiphilic Poly(N-vinylpyrrolidone) Nanoparticles Conjugated with DR5-Specific Antitumor Cytokine DR5-B for Targeted Delivery to Cancer Cells. Pharmaceutics 2021, 13, 1413. https://doi.org/10.3390/pharmaceutics13091413
Yagolovich A, Kuskov A, Kulikov P, Kurbanova L, Bagrov D, Artykov A, Gasparian M, Sizova S, Oleinikov V, Gileva A, et al. Amphiphilic Poly(N-vinylpyrrolidone) Nanoparticles Conjugated with DR5-Specific Antitumor Cytokine DR5-B for Targeted Delivery to Cancer Cells. Pharmaceutics. 2021; 13(9):1413. https://doi.org/10.3390/pharmaceutics13091413
Chicago/Turabian StyleYagolovich, Anne, Andrey Kuskov, Pavel Kulikov, Leily Kurbanova, Dmitry Bagrov, Artem Artykov, Marine Gasparian, Svetlana Sizova, Vladimir Oleinikov, Anastasia Gileva, and et al. 2021. "Amphiphilic Poly(N-vinylpyrrolidone) Nanoparticles Conjugated with DR5-Specific Antitumor Cytokine DR5-B for Targeted Delivery to Cancer Cells" Pharmaceutics 13, no. 9: 1413. https://doi.org/10.3390/pharmaceutics13091413
APA StyleYagolovich, A., Kuskov, A., Kulikov, P., Kurbanova, L., Bagrov, D., Artykov, A., Gasparian, M., Sizova, S., Oleinikov, V., Gileva, A., Kirpichnikov, M., Dolgikh, D., & Markvicheva, E. (2021). Amphiphilic Poly(N-vinylpyrrolidone) Nanoparticles Conjugated with DR5-Specific Antitumor Cytokine DR5-B for Targeted Delivery to Cancer Cells. Pharmaceutics, 13(9), 1413. https://doi.org/10.3390/pharmaceutics13091413