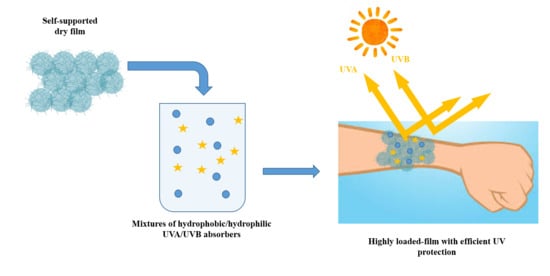
Spontaneously Self-Assembled Microgel Film as Co-Delivery System for Skincare Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Self-Assembled Microgel Films Formation
2.3. In Vitro Encapsulation of Active Molecules Mixtures
2.4. Characterization of Loaded Films
2.5. In Vitro Active Molecules Mixtures Release from Self-Assembled Microgel Films
3. Results and Discussion
3.1. Active Molecules Mixtures Encapsulation into Self-Assembled Microgel Films
3.2. In Vitro Release of Mixtures from Self-Assembled Microgel Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shalini, S.; Hansen, C.E.; Lyon, L.A. Microgel Mechanics in Biomaterial Design. Acc. Chem. Res. 2014, 47, 2426–2434. [Google Scholar]
- Elnaggar, Y.S.R.; El-Refaie, W.M.; El-Massik, M.A.; Abdallah, O.Y. Lecithin-based nanostructured gels for skin delivery: An update on state of art and recent applications. J. Control Release 2014, 180, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Serpe, M.J.; Yarmey, K.A.; Nolan, C.M.; Lyon, L.A. Doxorubicin uptake and release from microgel thin films. Biomacromolecules 2005, 6, 408–413. [Google Scholar] [CrossRef]
- Pérez-Guzmán, C.J.; Castro-Muñoz, R. A Review of Zein as Potential Biopolymer for Tissue Engineering and Nanotechnological Applications. Processes 2020, 8, 1376. [Google Scholar] [CrossRef]
- Biswal, T. Biopolymers for tissue engineering applications: A review. Mater. Today Proc. 2021, 41, 397–402. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New perspective. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, W.-F.; Susha, A.S.; Rogach, A.L. Multicompartment Microgel Beads for Co-Delivery of Multiple Drugs at Individual Release Rates. ACS Appl. Mater. Interfaces 2016, 8, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.-Y.; Zhang, R.; Du, F.-S.; Liang, D.-H.; Li, Z.-C. Multi-responsive nanogels containing motifs of ortho ester, oligo (ethylene glycol) and disulfide linkage as carriers of hydrophobic anti-cancer drugs. J. Control. Release 2011, 152, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Khanal, A.; Bui, M.-P.N.; Seo, S.S. Microgel-encapsulated methylene blue for the treatment of breast cancer cells by photodynamic therapy. J. Breast Cancer 2014, 17, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Costa, D.; Valente, A.J.M.; Miguel, M.G.; Queiroz, J. Plasmid DNA microgels for drug/gene co-delivery: A promising approach for cancer therapy. Colloids Sur. A Physicochem. Eng. Asp. 2014, 442, 181–190. [Google Scholar] [CrossRef]
- Qiao, H.; Chen, X.; Wang, Q.; Zhang, J.; Huang, D.; Chen, E.; Qian, H.; Zhang, Y.; Tang, Q.; Chen, W. Tumor localization of oncolytic adenovirus assisted by pH-degradable microgels with JQ1-mediated boosting replication and PD-L1 suppression for enhanced cancer therapy. Biomater. Sci. 2020, 8, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qian, H.; Qiao, H.; Dong, B.; Chen, E.; Huang, D.; Wang, T.; Chen, W. Tumor-Adhesive and pH-Degradable Microgels by Microfluidics and Photo-Cross-Linking for Efficient Antiangiogenesis and Enhanced Cancer Chemotherapy. Biomacromolecules 2020, 21, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sarker, A.K.; Islam, M.R.; Li, X.; Lu, Z.; Serpe, M.J. Poly(N-isopropylacrylamide) microgel-based assemblies. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3004–3020. [Google Scholar] [CrossRef]
- Li, X.; Serpe, M.J. Understanding and controlling the self-folding behavior of poly(N-isopropylacrylamide) microgel-based devices. Adv. Funct. Mater. 2014, 24, 4119–4126. [Google Scholar] [CrossRef]
- Ramos, J.; Imaz, A.; Forcada, J. Temperature-sensitive nanogels: Poly(N-vinylcaprolactam) versus poly(N-isopropylacrylamide). Polym. Chem. 2012, 3, 852–856. [Google Scholar] [CrossRef]
- Dieuzy, E.; Aguirre, G.; Auguste, S.; Chougrani, K.; Alard, V.; Billon, L.; Derail, C. Microstructure-driven self-assembly and rheological properties of multi-responsive soft microgel suspensions. J. Colloid Interface Sci. 2021, 581, 806–815. [Google Scholar] [CrossRef]
- Dieuzy, E.; Auguste, S.; Chougrani, K.; Alard, V.; Billon, L.; Derail, C. Microgel structure-driven linear and non-linear mechanical properties of self-assembled microgel films. Colloids Surf. A. Physicochem. Eng. Asp. 2021, 613, 126082–126093. [Google Scholar] [CrossRef]
- Boularas, M.; Radji, S.; Gombart, E.; Alard, V.; Tranchant, J.-F.; Billon, L. Funtional film by trigger-free self-assembly of adhesive soft microgels at skin temperature. Mater. Des. 2018, 147, 19–27. [Google Scholar] [CrossRef]
- Aguirre, A.; Khoukh, A.; Taboada, P.; Chougrani, K.; Alard, V.; Billon, L. Smart self-assembled microgel films as encapsulating carriers for UV-absorbing molecules. Polym. Chem. 2018, 9, 1155–1159. [Google Scholar] [CrossRef]
- Aguirre, G.; Alard, V.; Billon, L. Microgels for the Delivery of Cosmetic Active Organic Substances. European Patent EP17196940, 17 October 2017. [Google Scholar]
- Soltantabar, P.; Calubaquib, E.L.; Mostafavi, E.; Biewer, M.C.; Stefan, M.C. Enhancement of loading efficiency by coloading of doxorubicin and querceting in thermoresponsive polymeric micelles. Biomacromolecules 2020, 21, 1427–1436. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Nanocarrier mediated combination drug delivery for chemotherapy-Review. J. Drug Deliv. Sci. Technol. 2017, 39, 362–371. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y. Combination therapy based on nano codelivery for overcoming cancer drug resistance. Med. Drug Discov. 2020, 6, DI100024. [Google Scholar] [CrossRef]
- Boularas, M.; Deniau-Lejeune, E.; Alard, V.; Tranchant, J.-F.; Billon, L.; Save, M. Dual stimuli-responsive oligo (ethylene glycol)-based microgels: Insight into the role of internal structure in volume phase transitions and loading of magnetic nanoparticles to design stable thermoresponsive hybrid microgels. Polym. Chem. 2016, 7, 350–363. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Aguirre, A.; Khoukh, A.; Chougrani, K.; Alard, V.; Billon, L. Dual-responsive biocompatible microgels as high loaded cargo: Understanding of encapsulation/release driving forces by NMR NOESY. Polym. Chem. 2018, 9, 757–768. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Boateng, J.; Mani, J.; Kianfar, F. Improving drug loading of mucosal solvent cast films using a combination of hydrophilic polymers with amoxicillin and paracetamol as model drugs. BioMed Res. Int. 2013, 2013, 198137. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, Y.G.; Galgatte, U.C.; Chaudhari, P.D. Challenges in formulation evelopment of fast dissolving oral films. Indo Am. J. Pharm. Res. 2013, 3, 1746–1751. [Google Scholar]
- Perumal, V.A.; Govender, T.; Lutchman, D.; Mackraj, I. Investigating a new approach to film casting for enhanced drug content uniformity in polymeric film. Drug Dev. Ind. Pharm. 2008, 34, 1036–1047. [Google Scholar] [CrossRef]
- Gui, R.; Wang, Y.; Sun, J. Embedding fluorescent mesoporous silica nanoparticles into biocompatible nanogels for tumor cell imaging and thermo/pH-sensitive in vitro drug release. Colloids Surf. B 2014, 116, 518–525. [Google Scholar] [CrossRef]
- Xu, X.; Shan, G.R.; Pan, P. Controlled co-delivering of hydrophilic and hydrophobic drugs from thermosensitive and crystallizable copolymer nanoparticles. J. Appl. Polym. Sci. 2016, 133, 44132. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pikabea, A.; Villar-Alvarez, E.; Forcada, J.; Taboada, P. pH-controlled doxorubicin delivery from PDEAEMA-based nanogels. J. Mol. Liq. 2018, 266, 321–329. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Auffret, A.D.; Humphrey, M.J.; Stevents, H.N.; Eccleston, G.M. In vitro drug release studies of polymeric freeze-dried wafers and solvent-cast films using paracetamol as a model soluble drug. Int. J. Pharm. 2009, 378, 66–72. [Google Scholar] [CrossRef]
- Silva, T.N.; Reynaud, F.; Souza Picciani, P.H.; Holanda e Silva, K.G.; Nogueira Barradas, T. Chitosan-based films containing nanoemulsions of methyl salicylate:Formulation development, physical-chemical and in vitro drug release characterization. Int. J. Biol. Macromol. 2020, 164, 2558–2568. [Google Scholar] [CrossRef]



| Loaded Amount (mg/mgfilm) | Encapsulation Efficiencies (E.E.) % | |||||
|---|---|---|---|---|---|---|
| Mixture A | Mixture B | Mixture C | ||||
| DBBH | Benzophenone-4 | DBBH | Salicylic Acid | Benzophenone-4 | Salicylic Acid | |
| 0.5 | 23.0 ± 11.3 | 0 | 26.4 ± 9.1 | 2.8 ± 3.9 | 28.6 ± 1.3 | 51.4 ± 0.8 |
| 1 | 57.0 ± 2.0 | 19.0 ± 3.0 | 57.0 ± 5.3 | 21.50 ± 5.4 | 73.2 ± 0.4 | 87.4 ± 0.5 |
| Hydrophilic/ Hydrophobic Ratio | Encapsulation Efficiencies (E.E.) % | |||
|---|---|---|---|---|
| Mixture A | Mixture B | |||
| DBBH | Benzophenone-4 | DBBH | Salicylic Acid | |
| 3:1 | 73.0 ± 1.7 | 81.1 ± 0.4 | 80.5 ± 0.4 | 87.2 ± 0.2 |
| 9:1 | 57.3 ± 1.7 | 87.30 ± 0.6 | 83.10 ± 1.9 | 90.3 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguirre, G.; Taboada, P.; Billon, L. Spontaneously Self-Assembled Microgel Film as Co-Delivery System for Skincare Applications. Pharmaceutics 2021, 13, 1422. https://doi.org/10.3390/pharmaceutics13091422
Aguirre G, Taboada P, Billon L. Spontaneously Self-Assembled Microgel Film as Co-Delivery System for Skincare Applications. Pharmaceutics. 2021; 13(9):1422. https://doi.org/10.3390/pharmaceutics13091422
Chicago/Turabian StyleAguirre, Garbine, Pablo Taboada, and Laurent Billon. 2021. "Spontaneously Self-Assembled Microgel Film as Co-Delivery System for Skincare Applications" Pharmaceutics 13, no. 9: 1422. https://doi.org/10.3390/pharmaceutics13091422
APA StyleAguirre, G., Taboada, P., & Billon, L. (2021). Spontaneously Self-Assembled Microgel Film as Co-Delivery System for Skincare Applications. Pharmaceutics, 13(9), 1422. https://doi.org/10.3390/pharmaceutics13091422