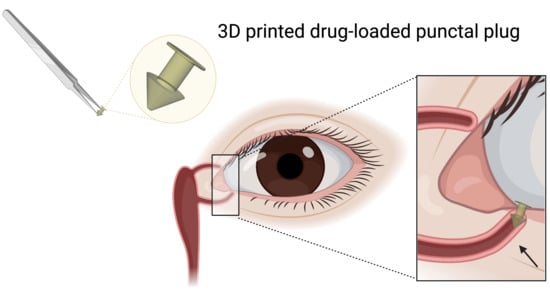
3D Printed Punctal Plugs for Controlled Ocular Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Design of the Punctal Plug
2.2.2. Preparation of Photopolymer Solutions
2.2.3. Printing Process
2.2.4. UV-Visible Spectrophotometry
2.2.5. X-ray Powder Diffraction (XRPD)
2.2.6. Differential Scanning Calorimetry (DSC)
2.2.7. Device Morphology
2.2.8. Scanning Electron Microscopy (SEM)
2.2.9. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.10. In Vitro Drug Release from the Devices
2.2.11. Determination of Drug Content
2.2.12. Cytocompatibility Studies
3. Results and Discussion
3.1. DLP 3D Printing
3.2. Physical Characterisation of the 3D Printed Formulations
3.3. Drug Release Studies
3.4. Cytocompatibility Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Awwad, S.; Ahmed, A.H.A.M.; Sharma, G.; Heng, J.S.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of pharmacology in the eye. Br. J. Pharmacol. 2017, 174, 4205–4223. [Google Scholar] [CrossRef]
- Uchino, M.; Schaumberg, D.A. Dry eye disease: Impact on quality of life and vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouen, P.A.; White, M.L. Dry eye disease: Prevalence, assessment, and management. Home Healthc. Now 2018, 36, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular drug delivery: Present innovations and future challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Yellepeddi, V.K.; Sheshala, R.; McMillan, H.; Gujral, C.; Jones, D.; Raghu Raj Singh, T. Punctal plug: A medical device to treat dry eye syndrome and for sustained drug delivery to the eye. Drug Discov. Today 2015, 20, 884–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Lorenzo, C.; Anguiano-Igea, S.; Varela-García, A.; Vivero-Lopez, M.; Concheiro, A. Bioinspired hydrogels for drug-eluting contact lenses. Acta Biomater. 2019, 84, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef]
- Huang, D.; Chen, Y.-S.; Rupenthal, I.D. Overcoming ocular drug delivery barriers through the use of physical forces. Adv. Drug Deliv. Rev. 2018, 126, 96–112. [Google Scholar] [CrossRef]
- Jung, J.H.; Chiang, B.; Grossniklaus, H.E.; Prausnitz, M.R. Ocular drug delivery targeted by iontophoresis in the suprachoroidal space using a microneedle. J. Control. Release 2018, 277, 14–22. [Google Scholar] [CrossRef]
- Than, A.; Liu, C.; Chang, H.; Duong, P.K.; Cheung, C.M.G.; Xu, C.; Wang, X.; Chen, P. Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nat. Commun. 2018, 9, 4433. [Google Scholar] [CrossRef] [Green Version]
- Thakur Singh, R.R.; Tekko, I.; McAvoy, K.; McMillan, H.; Jones, D.; Donnelly, R.F. Minimally invasive microneedles for ocular drug delivery. Expert Opin. Drug Deliv. 2017, 14, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A comprehensive review on contact lens for ophthalmic drug delivery. J. Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef]
- Yuan, X.; Marcano, D.C.; Shin, C.S.; Hua, X.; Isenhart, L.C.; Pflugfelder, S.C.; Acharya, G. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano 2015, 9, 1749–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coursey, T.G.; Henriksson, J.T.; Marcano, D.C.; Shin, C.S.; Isenhart, L.C.; Ahmed, F.; De Paiva, C.S.; Pflugfelder, S.C.; Acharya, G. Dexamethasone nanowafer as an effective therapy for dry eye disease. J. Control. Release 2015, 213, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, C.; Chauhan, A. Ophthalmic delivery of cyclosporine A by punctal plugs. J. Control. Release 2011, 150, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, C.; Ning, Q.; Gao, Q.; Gao, C.; Gou, Z.; Ye, J. A new strategy to sustained release of ocular drugs by one-step drug-loaded microcapsule manufacturing in hydrogel punctal plugs. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2173–2184. [Google Scholar] [CrossRef]
- Jehangir, N.; Bever, G.; Mahmood, S.M.; Moshirfar, M. Comprehensive Review of the Literature on Existing Punctal Plugs for the Management of Dry Eye Disease. J. Ophthalmol. 2016, 2016, 9312340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ervin, A.M.; Law, A.; Pucker, A.D. Punctal occlusion for dry eye syndrome. Cochrane Database Syst. Rev. 2017, 6, CD006775. [Google Scholar] [CrossRef] [PubMed]
- Goto, E.; Yagi, Y.; Kaido, M.; Matsumoto, Y.; Konomi, K.; Tsubota, K. Improved functional visual acuity after punctal occlusion in dry eye patients. Am. J. Ophthalmol. 2003, 135, 704–705. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.C.; Sheikh, A.A. 3D printing in aerospace and its long-term sustainability. Virtual Phys. Prototyp. 2015, 10, 175–185. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Yan, L.; Huang, D.; Lin, L.-Y. Extrusion-based food printing for digitalized food design and nutrition control. J. Food Eng. 2018, 220, 1–11. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, M.; Bhandari, B. Recent development in 3D food printing. Crit. Rev. Food Sci. Nutr. 2017, 57, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Wehner, M.; Truby, R.L.; Fitzgerald, D.J.; Mosadegh, B.; Whitesides, G.M.; Lewis, J.A.; Wood, R.J. An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature 2016, 536, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [Green Version]
- Prendergast, M.E.; Burdick, J.A. Recent advances in enabling technologies in 3D printing for precision medicine. Adv. Mater. 2020, 32, 1902516. [Google Scholar] [CrossRef]
- Elbadawi, M.; McCoubrey, L.E.; Gavins, F.K.H.; Ong, J.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Disrupting 3D printing of medicines with machine learning. Trends Pharmacol. Sci. 2021, 42, 745–757. [Google Scholar] [CrossRef]
- Isreb, A.; Baj, K.; Wojsz, M.; Isreb, M.; Peak, M.; Alhnan, M.A. 3D printed oral theophylline doses with innovative ‘radiator-like’design: Impact of polyethylene oxide (PEO) molecular weight. Int. J. Pharm. 2019, 564, 98–105. [Google Scholar] [CrossRef]
- Sadia, M.; Arafat, B.; Ahmed, W.; Forbes, R.T.; Alhnan, M.A. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J. Control. Release 2018, 269, 355–363. [Google Scholar] [CrossRef]
- Awad, A.; Yao, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Tablets (Printlets) with Braille and Moon Patterns for Visually Impaired Patients. Pharmaceutics 2020, 12, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melocchi, A.; Uboldi, M.; Parietti, F.; Cerea, M.; Foppoli, A.; Palugan, L.; Gazzaniga, A.; Maroni, A.; Zema, L. Lego-inspired capsular devices for the development of personalized dietary supplements: Proof of concept with multimodal release of caffeine. J. Pharm. Sci. 2020, 109, 1990–1999. [Google Scholar] [CrossRef] [PubMed]
- Rycerz, K.; Stepien, K.A.; Czapiewska, M.; Arafat, B.T.; Habashy, R.; Isreb, A.; Peak, M.; Alhnan, M.A. Embedded 3D printing of novel bespoke soft dosage form concept for pediatrics. Pharmaceutics 2019, 11, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, B.C.; Isreb, A.; Forbes, R.T.; Dores, F.; Habashy, R.; Petit, J.-B.; Alhnan, M.A.; Oga, E.F. ‘Temporary Plasticiser’: A novel solution to fabricate 3D printed patient-centred cardiovascular ‘Polypill’architectures. Eur. J. Pharm. Biopharm. 2019, 135, 94–103. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Xu, X.; Robles-Martinez, P.; Madla, C.M.; Joubert, F.; Goyanes, A.; Basit, A.W.; Gaisford, S. Stereolithography (SLA) 3D printing of an antihypertensive polyprintlet: Case study of an unexpected photopolymer-drug reaction. Addit. Manuf. 2020, 33, 101071. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Trenfield, S.J.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology. Pharmaceutics 2019, 11, 148. [Google Scholar] [CrossRef] [Green Version]
- Pereira, B.C.; Isreb, A.; Isreb, M.; Forbes, R.T.; Oga, E.F.; Alhnan, M.A. Computer-aided Drug Design: Additive Manufacturing of a Point-of-Care “Polypill:” Fabrication of Concept Capsules of Complex Geometry with Bespoke Release against Cardiovascular Disease (Adv. Healthcare Mater. 13/2020). Adv. Healthc. Mater. 2020, 9, 2070042. [Google Scholar] [CrossRef]
- Tan, Y.J.N.; Yong, W.P.; Kochhar, J.S.; Khanolkar, J.; Yao, X.; Sun, Y.; Ao, C.K.; Soh, S. On-demand fully customizable drug tablets via 3D printing technology for personalized medicine. J. Control. Release 2020, 322, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Muñiz Castro, B.; Elbadawi, M.; Ong, J.J.; Pollard, T.; Song, Z.; Gaisford, S.; Pérez, G.; Basit, A.W.; Cabalar, P.; Goyanes, A. Machine learning predicts 3D printing performance of over 900 drug delivery systems. J. Control. Release 2021, 337, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Gioumouxouzis, C.I.; Katsamenis, O.L.; Bouropoulos, N.; Fatouros, D.G. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Fina, F.; Goyanes, A.; Rowland, M.; Gaisford, S.; W Basit, A. 3D Printing of Tunable Zero-Order Release Printlets. Polymers 2020, 12, 1769. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Carmone, S.; Brambilla, D.; Leroux, J.-C. 3D printing of a wearable personalized oral delivery device: A first-in-human study. Sci. Adv. 2018, 4, 2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melocchi, A.; Inverardi, N.; Uboldi, M.; Baldi, F.; Maroni, A.; Pandini, S.; Briatico-Vangosa, F.; Zema, L.; Gazzaniga, A. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly(vinyl alcohol): Design concept and 4D printing feasibility. Int. J. Pharm. 2019, 559, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Uddin, M.J.; Scoutaris, N.; Economidou, S.N.; Giraud, C.; Chowdhry, B.Z.; Donnelly, R.F.; Douroumis, D. 3D printed microneedles for anticancer therapy of skin tumours. Mater. Sci. Eng. C 2020, 107, 110248. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, X.; Jin, Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int. J. Pharm. 2018, 539, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.-W.; Lee, K.-X.A.; Yeong, W.Y.; Shen, Y.-F. Vat polymerization-based bioprinting—Process, materials, applications and regulatory challenges. Biofabrication 2020, 12, 022001. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J. Control. Release 2021, 329, 743–757. [Google Scholar] [CrossRef]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef]
- Karakurt, I.; Aydoğdu, A.; Çıkrıkcı, S.; Orozco, J.; Lin, L. Stereolithography (SLA) 3D Printing of Ascorbic Acid Loaded Hydrogels: A Controlled Release Study. Int. J. Pharm. 2020, 584, 119428. [Google Scholar] [CrossRef]
- Vaut, L.; Juszczyk, J.J.; Kamguyan, K.; Jensen, K.E.; Tosello, G.; Boisen, A. 3D Printing of Reservoir Devices for Oral Drug Delivery: From Concept to Functionality through Design Improvement for Enhanced Mucoadhesion. ACS Biomater. Sci. Eng. 2020, 6, 2478–2486. [Google Scholar] [CrossRef]
- Lim, S.H.; Ng, J.Y.; Kang, L. Three-dimensional printing of a microneedle array on personalized curved surfaces for dual-pronged treatment of trigger finger. Biofabrication 2017, 9, 015010. [Google Scholar] [CrossRef] [PubMed]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, A.R.; Caudill, C.L.; Tumbleston, J.R.; Bloomquist, C.J.; Moga, K.A.; Ermoshkin, A.; Shirvanyants, D.; Mecham, S.J.; Luft, J.C.; DeSimone, J.M. Single-step fabrication of computationally designed microneedles by continuous liquid interface production. PLoS ONE 2016, 11, e0162518. [Google Scholar] [CrossRef]
- Janusziewicz, R.; Mecham, S.J.; Olson, K.R.; Benhabbour, S.R. Design and Characterization of a Novel Series of Geometrically Complex Intravaginal Rings with Digital Light Synthesis. Adv. Mater. Technol. 2020, 5, 2000261. [Google Scholar] [CrossRef]
- Yue, J.; Zhao, P.; Gerasimov, J.Y.; van de Lagemaat, M.; Grotenhuis, A.; Rustema-Abbing, M.; van der Mei, H.C.; Busscher, H.J.; Herrmann, A.; Ren, Y. 3D-Printable antimicrobial composite resins. Adv. Funct. Mater. 2015, 25, 6756–6767. [Google Scholar] [CrossRef]
- Xu, X.; Goyanes, A.; Trenfield, S.J.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Gaisford, S.; Basit, A.W. Stereolithography (SLA) 3D printing of a bladder device for intravesical drug delivery. Mater. Sci. Eng. C 2021, 120, 111773. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Lopez, M.; Xu, X.; Muras, A.; Otero, A.; Concheiro, A.; Gaisford, S.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Anti-biofilm multi drug-loaded 3d printed hearing aids. Mater. Sci. Eng. C 2021, 119, 111606. [Google Scholar] [CrossRef]
- Zidan, G.; Greene, C.A.; Etxabide, A.; Rupenthal, I.D.; Seyfoddin, A. Gelatine-based drug-eluting bandage contact lenses: Effect of PEGDA concentration and manufacturing technique. Int. J. Pharm. 2021, 599, 120452. [Google Scholar] [CrossRef]
- Patane, M.A.; Cohen, A.; From, S.; Torkildsen, G.; Welch, D.; Ousler, G.W., III. Ocular iontophoresis of EGP-437 (dexamethasone phosphate) in dry eye patients: Results of a randomized clinical trial. Clin. Ophthalmol. 2011, 5, 633. [Google Scholar]
- Angkawinitwong, U.; Awwad, S.; Khaw, P.T.; Brocchini, S.; Williams, G.R. Electrospun formulations of bevacizumab for sustained release in the eye. Acta Biomater. 2017, 64, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Shin, M.; Kim, B.; Park, J.W.; Lee, H. A visible light-curable yet visible wavelength-transparent resin for stereolithography 3D printing. NPG Asia Mater. 2018, 10, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Ahn, D.; Stevens, L.M.; Zhou, K.; Page, Z.A. Rapid high-resolution visible light 3D printing. ACS Cent. Sci. 2020, 6, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Madžarević, M.; Ibrić, S. Evaluation of exposure time and visible light irradiation in LCD 3D printing of ibuprofen extended release tablets. Eur. J. Pharm. Sci. 2021, 158, 105688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef]
- Urrios, A.; Parra-Cabrera, C.; Bhattacharjee, N.; Gonzalez-Suarez, A.M.; Rigat-Brugarolas, L.G.; Nallapatti, U.; Samitier, J.; DeForest, C.A.; Posas, F.; Garcia-Cordero, J.L. 3D-printing of transparent bio-microfluidic devices in PEG-DA. Lab Chip 2016, 16, 2287–2294. [Google Scholar] [CrossRef]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [Green Version]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Breloy, L.; Ouarabi, C.A.; Brosseau, A.; Dubot, P.; Brezova, V.; Abbad Andaloussi, S.; Malval, J.-P.; Versace, D.-L. β-Carotene/limonene derivatives/eugenol: Green synthesis of antibacterial coatings under visible-light exposure. ACS Sustain. Chem. Eng. 2019, 7, 19591–19604. [Google Scholar] [CrossRef]
- Field, J.; Haycock, J.W.; Boissonade, F.M.; Claeyssens, F. A Tuneable, Photocurable, Poly (Caprolactone)-Based Resin for Tissue Engineering—Synthesis, Characterisation and Use in Stereolithography. Molecules 2021, 26, 1199. [Google Scholar] [CrossRef]
- Li, D.; Guo, G.; Fan, R.; Liang, J.; Deng, X.; Luo, F.; Qian, Z. PLA/F68/Dexamethasone implants prepared by hot-melt extrusion for controlled release of anti-inflammatory drug to implantable medical devices: I. Preparation, characterization and hydrolytic degradation study. Int. J. Pharm. 2013, 441, 365–372. [Google Scholar] [CrossRef]
- da Silva, G.R.; da Silva-Cunha Jr, A.; Behar-Cohen, F.; Ayres, E.; Orefice, R.L. Biodegradable polyurethane nanocomposites containing dexamethasone for ocular route. Mater. Sci. Eng. C 2011, 31, 414–422. [Google Scholar] [CrossRef]
- Clark, E.A.; Alexander, M.R.; Irvine, D.J.; Roberts, C.J.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Hague, R.J.; Tuck, C.J.; Wildman, R.D. 3D printing of tablets using inkjet with UV photoinitiation. Int. J. Pharm. 2017, 529, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Visentin, A.F.; Dong, T.; Poli, J.; Panzer, M.J. Rapid, microwave-assisted thermal polymerization of poly (ethylene glycol) diacrylate-supported ionogels. J. Mater. Chem. A 2014, 2, 7723–7726. [Google Scholar] [CrossRef] [Green Version]
- Krkobabić, M.; Medarević, D.; Cvijić, S.; Grujić, B.; Ibrić, S. Hydrophilic excipients in digital light processing (DLP) printing of sustained release tablets: Impact on internal structure and drug dissolution rate. Int. J. Pharm. 2019, 572, 118790. [Google Scholar] [CrossRef]
- Lin, H.; Kai, T.; Freeman, B.D.; Kalakkunnath, S.; Kalika, D.S. The effect of cross-linking on gas permeability in cross-linked poly (ethylene glycol diacrylate). Macromolecules 2005, 38, 8381–8393. [Google Scholar] [CrossRef]
- Awwad, S.; Day, R.M.; Khaw, P.T.; Brocchini, S.; Fadda, H.M. Sustained release ophthalmic dexamethasone: In vitro in vivo correlations derived from the PK-Eye. Int. J. Pharm. 2017, 522, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- ISO, I. 10993–5: 2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009.
- Xue, D.; Wang, Y.; Zhang, J.; Mei, D.; Wang, Y.; Chen, S. Projection-based 3D printing of cell patterning scaffolds with multiscale channels. ACS Appl. Mater. Interfaces 2018, 10, 19428–19435. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.F.; Puga, A.M.; Díaz-Gomez, L.; Concheiro, A.; Busch, D.H.; Alvarez-Lorenzo, C. Additive manufacturing of scaffolds with dexamethasone controlled release for enhanced bone regeneration. Int. J. Pharm. 2015, 496, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Farto-Vaamonde, X.; Auriemma, G.; Aquino, R.P.; Concheiro, A.; Alvarez-Lorenzo, C. Post-manufacture loading of filaments and 3D printed PLA scaffolds with prednisolone and dexamethasone for tissue regeneration applications. Eur. J. Pharm. Biopharm. 2019, 141, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, Y.; Yang, L.; Wei, Y.; Wang, X.; Wang, Z.; Tao, L. Cytotoxicity study of polyethylene glycol derivatives. RSC Adv. 2017, 7, 18252–18259. [Google Scholar] [CrossRef] [Green Version]
- Roberts, C.W.; Carniglia, P.E.; Brazzo, B.G. Comparison of topical cyclosporine, punctal occlusion, and a combination for the treatment of dry eye. Cornea 2007, 26, 805–809. [Google Scholar] [CrossRef]
- Tai, M.-C.; Cosar, C.B.; Cohen, E.J.; Rapuano, C.J.; Laibson, P.R. The clinical efficacy of silicone punctal plug therapy. Cornea 2002, 21, 135–139. [Google Scholar] [CrossRef]
- Singh, R.B.; Ichhpujani, P.; Thakur, S.; Jindal, S. Promising therapeutic drug delivery systems for glaucoma: A comprehensive review. Ther. Adv. Ophthalmol. 2020, 12, 2515841420905740. [Google Scholar] [CrossRef] [Green Version]
- Chee, S.-P. Moxifloxacin punctum plug for sustained drug delivery. J. Ocul. Pharmacol. Ther. 2012, 28, 340–349. [Google Scholar] [CrossRef]










| Formulations | Dexamethasone (%) | Irgacure 819 (%) | β-Carotene (%) | PEGDA (%) | PEG 400 (%) |
|---|---|---|---|---|---|
| D10 | 10.0 | 2.0 | 1.0 | 87.0 | 0.0 |
| D20 | 20.0 | 2.0 | 1.0 | 77.0 | 0.0 |
| D10PEG | 10.0 | 2.0 | 1.0 | 69.6 | 17.4 |
| D20PEG | 20.0 | 2.0 | 1.0 | 61.6 | 15.4 |
| Formulations | Weight (mg ± SD) | Length (mm ± SD) |
|---|---|---|
| D10 | 0.86 ± 0.03 | 1.92 ± 0.03 |
| D20 | 1.50 ± 0.03 | 2.02 ± 0.02 |
| D10PEG | 0.59 ± 0.05 | 1.81 ± 0.05 |
| D20PEG | 1.39 ± 0.03 | 2.04 ± 0.01 |
| Formulations | Drug Loading (%, w/w ± SD) | Dose (µg) | |
|---|---|---|---|
| Photopolymer Solution | DLP 3D Printed Punctal Plugs | ||
| D10 | 11.9 ± 0.3 | 9.8 ± 0.5 | 84.4 |
| D20 | 20.2 ± 1.6 | 20.3 ± 2.1 | 305.6 |
| D10PEG | 10.6 ± 0.1 | 10.0 ± 0.6 | 59.0 |
| D20PEG | 19.4 ± 2.1 | 21.2 ± 1.4 | 294.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Awwad, S.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Brocchini, S.; Gaisford, S.; Goyanes, A.; Basit, A.W. 3D Printed Punctal Plugs for Controlled Ocular Drug Delivery. Pharmaceutics 2021, 13, 1421. https://doi.org/10.3390/pharmaceutics13091421
Xu X, Awwad S, Diaz-Gomez L, Alvarez-Lorenzo C, Brocchini S, Gaisford S, Goyanes A, Basit AW. 3D Printed Punctal Plugs for Controlled Ocular Drug Delivery. Pharmaceutics. 2021; 13(9):1421. https://doi.org/10.3390/pharmaceutics13091421
Chicago/Turabian StyleXu, Xiaoyan, Sahar Awwad, Luis Diaz-Gomez, Carmen Alvarez-Lorenzo, Steve Brocchini, Simon Gaisford, Alvaro Goyanes, and Abdul W. Basit. 2021. "3D Printed Punctal Plugs for Controlled Ocular Drug Delivery" Pharmaceutics 13, no. 9: 1421. https://doi.org/10.3390/pharmaceutics13091421
APA StyleXu, X., Awwad, S., Diaz-Gomez, L., Alvarez-Lorenzo, C., Brocchini, S., Gaisford, S., Goyanes, A., & Basit, A. W. (2021). 3D Printed Punctal Plugs for Controlled Ocular Drug Delivery. Pharmaceutics, 13(9), 1421. https://doi.org/10.3390/pharmaceutics13091421