New Prospects in Neutering Male Animals Using Magnetic Nanoparticle Hyperthermia
Abstract
:1. Introduction
2. Materials and Methods
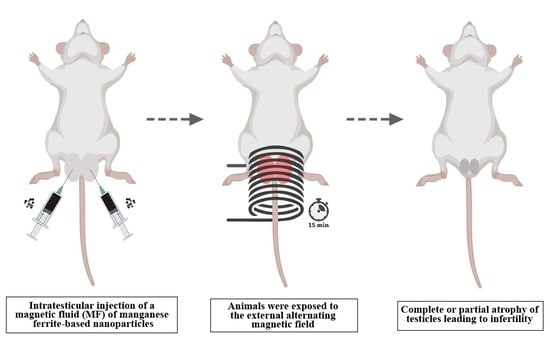
2.1. Magnetic Nanoparticles, Animals and Experimental Design
2.2. Experimental Procedures
2.3. Histological Processing
2.4. MNP Detection and Quantification by Ferromagnetic Resonance (FMR)
2.5. Statistical Analysis
3. Results
3.1. Magnetic Nanoparticle Hyperthermia
3.2. Clinical Observations
3.3. Macroscopic Testicular Analysis
3.4. Testicular Histopathology
3.5. Analysis of Vital Organs and MNP Quantification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barkalina, N.; Charalambous, C.; Jones, C.; Coward, K. Nanotechnology in reproductive medicine: Emerging applications of nanomaterials. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 921–938. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, X.; Ran, X.; Ju, R.; Ren, J.; Qu, X. Single-layer tungsten oxide as intelligent photo-responsive nanoagents for permanent male sterilization. Biomaterials 2015, 69, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, X.; Du, Y.; Ren, J.; Qu, X. Using Plasmonic Copper Sulfide Nanocrystals as Smart Light-Driven Sterilants. ACS Nano 2015, 9, 10335–10346. [Google Scholar] [CrossRef]
- Yostawonkul, J.; Surassmo, S.; Namdee, K.; Khongkow, M.; Boonthum, C.; Pagseesing, S.; Saengkrit, N.; Ruktanonchai, U.R.; Chatdarong, K.; Ponglowhapan, S.; et al. Nanocarrier-mediated delivery of α-mangostin for non-surgical castration of male animals. Sci. Rep. 2017, 7, 16234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, A.; Gamal, W.; Wu, X.; Yang, Y.; Olson, V.; D’Souza, M.J. Evaluation of an adjuvanted hydrogel-based pDNA nanoparticulate vaccine for rabies prevention and immunocontraception. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102049. [Google Scholar] [CrossRef]
- De Brito, J.L.; Lima, V.N.; Ansa, D.O.; Moya, S.E.; Morais, P.C.; Azevedo, R.B.; Lucci, C.M. Acute reproductive toxicology after intratesticular injection of silver nanoparticles (AgNPs) in Wistar rats. Nanotoxicology 2020, 7, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Sternheim, I. How Holland Became Free of Stray Dogs. DOGRESEARCH 2012. Available online: https://www.stray-afp.org/nl/wp-content/uploads/sites/2/2015/11/DR_Dutch_Straydogs1.pdf (accessed on 4 September 2021).
- Slater, M.R. The role of veterinary epidemiology in the study of free-roaming dogs and cats. Prev. Vet. Med. 2001, 48, 273–286. [Google Scholar] [CrossRef]
- Slater, M.R.; Shain, S. Feral cats: An overview. In The State of the Animals III, 1st ed.; Salem, D.J., Rowan, A.N., Eds.; Humane Society Press: Washington, DC, USA, 2005; pp. 43–53. [Google Scholar]
- FAO. Dog population management. In Proceedings of the FAO/WSPA/IZSAM Expert Meeting, Banna, Italy, 14–19 March 2011. Animal Production and Health Report 2014. No. 6. Rome. [Google Scholar]
- Jana, K.; Samanta, P.K. Evaluation of single intratesticular injection of calcium chloride for nonsurgical sterilization in adult albino rats. Contraception 2006, 73, 289–300. [Google Scholar] [CrossRef]
- Spehar, D.D.; Wolf, P.J. The Impact of an Integrated Program of Return-to-Field and Targeted Trap-Neuter-Return on Feline Intake and Euthanasia at a Municipal Animal Shelter. Animals 2018, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Spehar, D.D.; Wolf, P.J. Integrated Return-To-Field and Targeted Trap-Neuter-Vaccinate-Return Programs Result in Reductions of Feline Intake and Euthanasia at Six Municipal Animal Shelters. Front. Vet. Sci. 2019, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Eugster, S.; Schawalder, P.; Gaschen, F.; Boerlin, P. A Prospective Study of Postoperative Surgical Site Infections in Dogs and Cats. Vet. Surg. 2004, 33, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.; Wood, A. Non-surgical methods of contraception and sterilization. Theriogenology 2006, 66, 514–525. [Google Scholar] [CrossRef]
- Kustritz, R. Effects of Surgical Sterilization on Canine and Feline Health and on Society. Reprod. Dom. Anim. 2012, 47, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, J.K.; Bard, K.M.; Tucker, S.J.; Diskant, P.D.; Dingman, P.A. Perioperative mortality in cats and dogs undergoing spay or castration at a high-volume clinic. Vet. J. 2017, 224, 11–15. [Google Scholar] [CrossRef]
- Levy, J.K.; Crawford, C.; Appel, L.D.; Clifford, E.L. Comparison of intratesticular injection of zinc gluconate versus surgical castration to sterilize male dogs. Am. J. Vet. Res. 2008, 69, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, E.C.; Moura, M.R.; De Sá, M.J.; Junior, V.A.; Kastelic, J.P.; Douglas, R.H.; Junior, A.P. Permanent contraception of dogs induced with intratesticular injection of a Zinc Gluconate-based solution. Theriogenology 2012, 77, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, A.K.; Oliveira, E.C.; Tenorio, B.M.; Melo, C.C.; Nery, L.T.; Santos, F.A.; Alves, L.C.; Douglas, R.H.; Silva, V.A., Jr. Injection of a chemical castration agent, zinc gluconate, into the testes of cats results in the impairment of spermatogenesis: A potentially irreversible contraceptive approach for this species? Theriogenology 2014, 81, 230–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forzán, M.J.; Garde, E.; Pérez, G.E.; Vanderstichel, R.V. Necrosuppurative Orchitis and Scrotal Necrotizing Dermatitis Following Intratesticular Administration of Zinc Gluconate Neutralized With Arginine (EsterilSol) in 2 Mixed-Breed Dogs. Vet. Pathol. 2014, 51, 820–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Macêdo, S.R.; De Lima, L.A.; De Torres, S.M.; De Oliveira, V.V.; De Morais, R.N.; Peixoto, C.A.; Tenorio, B.M.; Da Silva Junior, V.A. Effects of intratesticular injection of zinc-based solution in rats in combination with anti-inflammatory and analgesic drugs during chemical sterilization. Vet. World 2018, 11, 649–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafatmah, D.; Mogheiseh, A.; Eshghi, D. Chemical sterilization with intratesticular administration of zinc gluconate in adult dogs: A preliminary report. Basic Clin. Androl. 2019, 29, 12. [Google Scholar] [CrossRef] [PubMed]
- Jana, K.; Samanta, P.K. Clinical Evaluation of Non-surgical Sterilization of Male Cats with Single Intra-testicular Injection of Calcium Chloride. BMC Vet. Res. 2011, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Leoci, R.; Aiudi, G.; Silvestre, F.; Lissner, E.A.; Marino, F.; Lacalandra, G.M. A dose-finding, long-term study on the use of calcium chloride in saline solution as a method of nonsurgical sterilization in dogs: Evaluation of the most effective concentration with the lowest risk. Acta Vet. Scand. 2014, 56, 63. [Google Scholar] [CrossRef] [Green Version]
- Kwak, B.K.; Lee, S. Intratesticular Injection of Hypertonic Saline: Non-Invasive Alternative Method for Animal Castration Model. Dev. Reprod. 2013, 17, 435–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineda, M.H.; Reimers, J.J.; Faulkner, L.C. The Disappearance of spermatozoa from the Ejaculates of Vasectomized Dogs. JAVMA 1977, 168, 502–503. [Google Scholar]
- Barnett, B.D. Chemical vasectomy of domestic dogs in the galapagos islands. Theriogenology 1985, 23, 499–509. [Google Scholar] [CrossRef]
- Fahim, M.S.; Wang, M.; Sutcu, M.F.; Fahim, Z.; Youngquist, R.S. Sterilization of dogs with intra-epididymal injection of zinc arginine. Contraception 1993, 47, 107–122. [Google Scholar] [CrossRef]
- Kastelic, J.P.; Wilde, R.E.; Rizzoto, G.; Thundathil, J.C. Hyperthermia and not hypoxia may reduce sperm motility and morphology following testicular hyperthermia. Vet. Med. 2017, 62, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Kastelic, J.P.; Wilde, R.E.; Bielli, A.; Genovese, P.; Rizzoto, P.; Thundathila, J. Hyperthermia is more important than hypoxia as a cause of disrupted spermatogenesis and abnormal sperm. Theriogenology 2019, 131, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Fahim, M.S.; Fahim, Z.; Der, R.; Hall, D.G.; Harman, J. Heat in male contraception (hot water 60 °C, infrared, microwave, and ultrasound). Contraception 1975, 11, 549–562. [Google Scholar] [CrossRef]
- Aktas, C.; Kanter, M. A morphological study on Leydig cells of scrotal hyperthermia applied rats in short-term. J. Mol. Hist. 2009, 40, 31–39. [Google Scholar] [CrossRef]
- Kanter, M.; Aktas, C. Effects of scrotal hyperthermia on Leydig cells in long-term: A histological, immunohistochemical and ultrastructural study in rats. J. Mol. Hist. 2009, 40, 123–130. [Google Scholar] [CrossRef]
- Tsuruta, J.K.; Dayton, P.A.; Gallippi, C.M.; Michael, G.O.; Streicker, M.A.; Gessner, R.C.; Gregory, T.S.; Silva, E.J.; Hamil, K.G.; Moser, G.J.; et al. Therapeutic ultrasound as a potential male contraceptive: Power, frequency and temperature required to deplete rat testes of meiotic cells and epididymides of sperm determined using a commercially available system. Reprod. Biol. Endocrinol. 2012, 10, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, A.S.; Dhaked, S.R.K.; Badar, A.; Khilwani, B.; Lohiya, N.K. Reproductive Functions and Toxicology Following Scrotal Ultrasound Therapy in Rats. IJSRBS 2020, 7, 15–24. [Google Scholar] [CrossRef]
- Leoci, R.; Aiudi, G.; Salvati, A.S.; Silvestre, F.; Binetti, F.; Lacalandra, G.M. Ultrasound as a Mechanical Method for Male Dog Contraception. Reprod. Domest. Anim. 2009, 44, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Leoci, R.; Aiudi, G.; Silvestre, F.; Lissner, E.A.; Marino, F.; Lacalandra, G.M. Therapeutic Ultrasound as a Potential Male Dog Contraceptive: Determination of the Most Effective Application Protocol. Reprod. Dom. Anim. 2015, 50, 712–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, H.F.; Capistrano, G.; Bakuzis, A.F. In vivo magnetic nanoparticle hyperthermia: A review on preclinical studies, low-field nanoheaters, noninvasive thermometry and computer simulations for treatment planning. Int. J. Hyperth. 2020, 37, 76–99. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.; Willi, T.; Rosen, Y.; Mefford, O.T.; Alexis, F. Targeted magnetic hyperthermia. Ther. Deliv. 2011, 2, 815–838. [Google Scholar] [CrossRef]
- Branquinho, L.C.; Carrião, M.S.; Costa, A.S.; Zufelato, N.; Sousa, M.H.; Miotto, R.; Ivkov, R.; Bakuzis, A.F. Effect of magnetic dipolar interactions on nanoparticle heating efficiency: Implications for cancer hyperthermia. Sci. Rep. 2013, 3, 2887. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, H.F.; Capistrano, G.; Mello, F.M.; Zufelato, N.; Silveira-Lacerda, E.; Bakuzis, A.F. Precise determination of the heat delivery during in vivo magnetic nanoparticle hyperthermia with infrared thermography. Phys. Med. Biol. 2017, 62, 4062–4082. [Google Scholar] [CrossRef]
- Capistrano, G.; Rodrigues, H.F.; Zufelato, N.; Gonçales, C.; Cardoso, C.G.; Silveira-Lacerda, E.P.; Bakuzis, A.F. Noninvasive intratumoral thermal dose determination during in vivo Magnetic nanoparticle hyperthermia: Combining surface temperature measurements and computer simulations. Int. J. Hyperther. 2020, 37, 120–140. [Google Scholar] [CrossRef]
- Sotocina, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebeck, J.C.; Wei, P.; Zhan, S.; Zhang, S.; et al. The rat grimace scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 2011, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Hickman, D.L.; Swan, M. Use of a body condition score technique to assess health status in a rat model of polycystic kidney disease. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 155–159. [Google Scholar] [PubMed]
- Chaves, S.B.; Lacava, L.M.; Lacava, Z.G.; Silva, O.; Pelegrini, F.; Buske, N.; Gansau, C.; Morais, P.C.; Azevedo, R.B. Light Microscopy and Magnetic Resonance Characterization of a DMSA-Coated Magnetic Fluid in Mice. IEEE Trans. Magn. 2002, 38, 3231–3233. [Google Scholar] [CrossRef]
- Louvandini, H.; McManus, C.; Martins, R.D.; Lucci, C.M.; Corrêa, P.S. Caracteíısticas Biométricas Testiculares em Carneiros Santa Inês Submetidos a Diferentes Regimes de Suplementaçãoo Protéica e Tratamentos anti-Helmíınticos. Ciênc. Anim. Bras. 2008, 9, 638–647. [Google Scholar]
- Wechalekar, H.; Setchell, B.P.; Pilkington, K.R.; Leigh, C.; Breed, W.G.; Peirce, E. Effects of whole-body heat on male germ cell development and sperm motility in the laboratory mouse. Reprod. Fertil. Dev. 2016, 28, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Eliçevik, M.; Tireli, G.; Sander, S.; Celayir, S. Plasma testosterone and estradiol levels in unilateral cryptorchidism. Arch. Neurol. 2006, 52, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Kut, C.; Zhang, Y.; Hedayati, M.; Zhou, H.; Cornejo, C.; Bordelon, D.; Mihalic, J.; Wabler, M.; Burghardt, E.; Gruettner, C.; et al. Preliminary study of injury from heating systemically delivered, nontargeted dextran–superparamagnetic iron oxide nanoparticles in mice. Nanomedicine 2012, 7, 1697–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Sun, C.; Wang, F.; Wang, Y.; Zhai, Y.; Liang, M.; Liu, W.; Liu, Z.; Wang, J.; Sun, F. Achieving a New Controllable Male Contraception by the Photothermal Effect of Gold Nanorods. Nano Lett. 2013, 13, 2477–2484. [Google Scholar] [CrossRef]
- Roberts, W.W.; Chan, D.Y.; Fried, N.M.; Wright, E.J.; Nicol, T.; Jarrett, T.W.; Kavoussi, L.R.; Solomon, S.B. High Intensity Focused Ultrasound Ablation of the Vas Deferens in a Canine Model. J. Urol. 2002, 167, 2613–2617. [Google Scholar] [CrossRef]
- Roberts, W.W.; Wright, E.J.; Fried, N.M.; Nicol, T.; Jarrett, T.W.; Kavoussi, L.R.; Solomon, S.B. High-Intensity Focused Ultrasound Ablation of the Epididymis in a Canine Model: A Potential Alternative to Vasectomy. J. Endourol. 2002, 16, 621–625. [Google Scholar] [CrossRef]
- Lacava, L.M.; Garcia, V.A.P.; Kuckelhaus, S.; Azevedo, R.B.; Sadeghiani, N.; Buske, N.; Morais, P.C.; Lacava, Z.G.M. Long-term retention of dextran-coated magnetite nanoparticles in the liver and spleen. J. Magn. Magn. Mater. 2004, 272–276, 2434–2435. [Google Scholar] [CrossRef]
- Garcia, M.P.; Parca, R.M.; Chaves, S.B.; Paulino Silva, L.; Santos, A.D.; Lacava, Z.G.M.; Morais, P.C.; Azevedo, R.B. Morphological analysis of mouse lungs after treatment with magnetite-based magnetic fluid stabilized with DMSA. J. Magn. Magn. Mater. 2005, 293, 277–282. [Google Scholar] [CrossRef]
- Gamarra Contreras, L.; Da Costa-Filho, A.J.; Mamani, J.B.; De Cassia Ruiz, R.; Pavon, L.F.; Sibov, T.T.; Vieira, E.D.; Silva, A.C.; Pontuschka, W.M.; Amaro, E., Jr. Ferromagnetic resonance for the quantification of superparamagnetic iron oxide nanoparticles in biological materials. Int. J. Nanomed. 2010, 5, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monge-Fuentes, V.; Garcia, M.P.; Tavares, M.C.H.; Valois, C.R.A.; Lima, E.C.D.; Teixeira, D.S.; Morais, P.C.; Tomaz, C.A.; Azevedo, R.B. Biodistribution and biocompatibility of DMSA stabilized maghemite magnetic nanoparticles in nonhuman primates (Cebus spp.). Nanomedicine 2011, 6, 1529–1544. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Fang, R.H.; Sailor, M.J.; Park, J.H. In vivo clearance and toxicity of monodisperse iron oxide nanocrystals. ACS Nano 2012, 6, 4947–4954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, A.; Gutiérrez, L.; Cáceres-Vélez, P.R.; Santos, D.; Chaves, S.B.; Fascineli, M.L.; Garcia, M.P.; Azevedo, R.B.; Morales, M.P. Biotransformation of magnetic nanoparticles as a function of coating in a rat model. Nanoscale 2015, 7, 16321. [Google Scholar] [CrossRef]
- Weissleder, R.; Stark, D.D.; Engelstad, B.L.; Bacon, B.A.; Compton, C.C.; White, D.L.; Jacobs, P.; Lewis, J. Superparamagnetic Iron Oxide: Pharmacokinetics and Toxicity. Am. J. Roentgenol. 1989, 152, 167–173. [Google Scholar] [CrossRef]
- Pouliquen, D.; Jeune, J.J.L.; Perdrisot, R.; Ermias, A.; Jallet, P. Iron oxide nanoparticles for use as an MRI contrast agent: Pharmacokinetics and metabolism. Magn. Reson. Imaging 1991, 9, 275–283. [Google Scholar] [CrossRef]
- Liu, T.; Choi, H.; Zhou, R.; Chen, I.W. Quantitative Evaluation of the Reticuloendothelial System Function with Dynamic MRI. PLoS ONE 2014, 10, e0122323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quini, C.C.; Próspero, A.G.; Calabresi, M.F.; Moretto, G.M.; Zufelato, N.; Krishnan, S.; Pina, D.R.; Oliveira, R.B.; Baffa, O.; Bakuzis, A.F.; et al. Real-Time Liver Uptake and Biodistribution of Magnetic Nanoparticles Determined by AC Biosusceptometry. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1519–1529. [Google Scholar] [CrossRef] [Green Version]
- Bourrinet, P.; Bengele, H.H.; Bonnemain, B.; Dencausse, A.; Idee, J.M.; Jacobs, P.M.; Lewis, J.M. Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agent. Investig. Radiol. 2006, 41, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zheng, J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [Green Version]
- McCord, J.M. Effects of Positive Iron Status at a Cellular Level. Nutr. Rev. 1996, 54, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Samanta, B.; Yan, H.; Fischer, N.O.; Shi, J.; Jerry, D.J.; Rotello, V.M. Protein-passivated Fe3O4 nanoparticles: Low toxicity and rapid heating for thermal therapy. J. Mater. Chem. 2008, 18, 1204–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 Saline Group, animals received an intratesticular injection of sterile saline solution.
Saline Group, animals received an intratesticular injection of sterile saline solution.  MF Group, animals received an intratesticular injection of the magnetic fluid.
MF Group, animals received an intratesticular injection of the magnetic fluid.  AC Group, animals were exposed to the external AC magnetic field only.
AC Group, animals were exposed to the external AC magnetic field only. 
 MNH Group, animals received an intratesticular injection of the magnetic fluid and were exposed to the external AC magnetic field. Animals were divided into 3 subgroups and evaluated: 7 (MNH-D7 Group), 28 (MNH-D28 Group), and 56 (MNH-D56 Group) days after the magnetic nanoparticle hyperthermia treatment.
MNH Group, animals received an intratesticular injection of the magnetic fluid and were exposed to the external AC magnetic field. Animals were divided into 3 subgroups and evaluated: 7 (MNH-D7 Group), 28 (MNH-D28 Group), and 56 (MNH-D56 Group) days after the magnetic nanoparticle hyperthermia treatment.
 Saline Group, animals received an intratesticular injection of sterile saline solution.
Saline Group, animals received an intratesticular injection of sterile saline solution.  MF Group, animals received an intratesticular injection of the magnetic fluid.
MF Group, animals received an intratesticular injection of the magnetic fluid.  AC Group, animals were exposed to the external AC magnetic field only.
AC Group, animals were exposed to the external AC magnetic field only. 
 MNH Group, animals received an intratesticular injection of the magnetic fluid and were exposed to the external AC magnetic field. Animals were divided into 3 subgroups and evaluated: 7 (MNH-D7 Group), 28 (MNH-D28 Group), and 56 (MNH-D56 Group) days after the magnetic nanoparticle hyperthermia treatment.
MNH Group, animals received an intratesticular injection of the magnetic fluid and were exposed to the external AC magnetic field. Animals were divided into 3 subgroups and evaluated: 7 (MNH-D7 Group), 28 (MNH-D28 Group), and 56 (MNH-D56 Group) days after the magnetic nanoparticle hyperthermia treatment.






| Organ | AC | MF | Saline | MNH-D7 | MNH-D28 | MNH-D56 |
|---|---|---|---|---|---|---|
| Liver | 3.56 ± 0.37 | 4.23 ± 0.13 | 4.36 ± 0.01 | 4.50 ± 0.74 | 3.94 ± 0.43 | 3.26 ± 0.16 |
| Spleen | 0.35 ± 0.02 | 0.63 ± 0.04 | 0.49 ± 0.08 | 0.57 ± 0.13 | 0.48 ± 0.08 | 0.35 ± 0.04 |
| Lungs | 0.65 ± 0.18 | 0.60 ± 0.01 | 0.63 ± 0.01 | 0.73 ± 0.21 | 0.52 ± 0.08 | 0.50 ± 0.06 |
| Kidneys | 0.46 ± 0.02 | 0.43 ± 0.01 | 0.41 ± 0.01 | 0.52 ± 0.19 | 0.43 ± 0.02 | 0.41 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jivago, J.L.P.R.; Brito, J.L.M.; Capistrano, G.; Vinícius-Araújo, M.; Lima Verde, E.; Bakuzis, A.F.; Souza, P.E.N.; Azevedo, R.B.; Lucci, C.M. New Prospects in Neutering Male Animals Using Magnetic Nanoparticle Hyperthermia. Pharmaceutics 2021, 13, 1465. https://doi.org/10.3390/pharmaceutics13091465
Jivago JLPR, Brito JLM, Capistrano G, Vinícius-Araújo M, Lima Verde E, Bakuzis AF, Souza PEN, Azevedo RB, Lucci CM. New Prospects in Neutering Male Animals Using Magnetic Nanoparticle Hyperthermia. Pharmaceutics. 2021; 13(9):1465. https://doi.org/10.3390/pharmaceutics13091465
Chicago/Turabian StyleJivago, José Luiz P. R., Juliana Lis Mendes Brito, Gustavo Capistrano, Marcus Vinícius-Araújo, Ediron Lima Verde, Andris Figueiroa Bakuzis, Paulo E. N. Souza, Ricardo Bentes Azevedo, and Carolina Madeira Lucci. 2021. "New Prospects in Neutering Male Animals Using Magnetic Nanoparticle Hyperthermia" Pharmaceutics 13, no. 9: 1465. https://doi.org/10.3390/pharmaceutics13091465
APA StyleJivago, J. L. P. R., Brito, J. L. M., Capistrano, G., Vinícius-Araújo, M., Lima Verde, E., Bakuzis, A. F., Souza, P. E. N., Azevedo, R. B., & Lucci, C. M. (2021). New Prospects in Neutering Male Animals Using Magnetic Nanoparticle Hyperthermia. Pharmaceutics, 13(9), 1465. https://doi.org/10.3390/pharmaceutics13091465