Tablet Splitting in Elderly Patients with Dementia: The Case of Quetiapine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection on Splitting Techniques
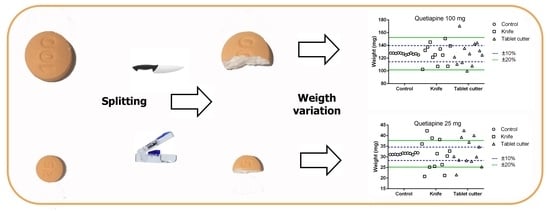
2.2. Effect of Splitting Technique on Tablets Weight and Dose
2.2.1. Materials
2.2.2. Sample Size
2.2.3. Sample Preparation and Data Collection
2.2.4. Statistical Analysis
3. Results
3.1. Data about Splitting Techniques
3.2. Effect of Splitting Technique on Tablet Weight and Dose
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kales, H.C.; Gitlin, L.N.; Lyketsos, C.G. Assessment and management of behavioral and psychological symptoms of dementia. BMJ 2015, 350, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, A.; Waldorff, F.B.; Waldemar, G. Impaired awareness of deficits and neuropsychiatric symptoms in early Alzheimer’s disease: The Danish Alzheimer Intervention Study (DAISY). J. Neuropsychiatry Clin. Neurosci. 2010, 22, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Koponen, M.; Taipale, H.; Tanskanen, A.; Tolppanen, A.-M.; Tiihonen, J.; Ahonen, R.; Hartikainen, S. Long-term use of antipsychotics among community-dwelling persons with Alzheimer’s disease: A nationwide register-based study. Eur. Neuropsychopharmacol. 2015, 25, 1706–1713. [Google Scholar] [CrossRef]
- Madhusoodanan, S.; Shah, P.; Brenner, R.; Gupta, S. Pharmacological treatment of the psychosis of Alzheimer’s disease: What is the best approach? CNS Drugs 2007, 21, 101–115. [Google Scholar] [CrossRef]
- Fauth, E.B.; Gibbons, A. Which behavioral and psychological symptoms of dementia are the most problematic? Variability by prevalence, intensity, distress ratings, and associations with caregiver depressive symptoms. Int. J. Geriatr. Psychiatry 2014, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Katona, C.; Livingston, G.; Cooper, C.; Ames, D.; Brodaty, H.; Chiu, E. International Psychogeriatric Association consensus statement on defining and measuring treatment benefits in dementia. Int. Psychogeriatr. 2007, 19, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.G.; Gauthier, S.; Cummings, J.L.; Brodaty, H.; Grossberg, G.T.; Robert, P.; Lyketsos, C.G. Management of agitation and aggression associated with alzheimer disease. Nat. Rev. Neurol. 2009, 5, 245–255. [Google Scholar] [CrossRef]
- Mascarenhas Starling, F.; Medeiros-Souza, P.; Francisco De Camargos, E.; Ferreira, F.; Rodrigues Silva, A.; Homem-De-Mello, M. Tablet Splitting of Psychotropic Drugs for Patients with Dementia: A Pharmacoepidemiologic Study in a Brazilian Sample. Clin. Ther. 2015, 37, 2332–2338. [Google Scholar] [CrossRef]
- Sestili, M.; Ferrara, L.; Logrippo, S.; Ganzetti, R. Detection of medication errors in hospital discharge communications of patients on enteral nutrition. Nutr. Ther. Metab. 2014, 32, 152–154. [Google Scholar] [CrossRef]
- Fusco, S.; Cariati, D.; Schepisi, R.; Ganzetti, R.; Sestili, M.; David, S.; Ferrara, L.; Liuzzi Gatto, M.; Vena, S.; Corsonello, A.; et al. Management of oral drug therapy in elderly patients with dysphagia. J. Gerontol. Geriatr. 2016, 64, 9–20. [Google Scholar]
- Logrippo, S.; Ricci, G.; Sestili, M.; Cespi, M.; Ferrara, L.; Palmieri, G.F.; Ganzetti, R.; Bonacucina, G.; Blasi, P. Oral drug therapy in elderly with dysphagia: Between a rock and a hard place! Clin. Interv. Aging 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.W.; Varker, A.S.; Karlage, K.; Myrdal, P.B. Analysis of drug content and weight uniformity for half-tablets of 6 commonly split medications. J. Manag. Care Pharm. 2009, 15, 253–261. [Google Scholar] [CrossRef]
- Van Riet-Nales, D.A.; Doeve, M.E.; Nicia, A.E.; Teerenstra, S.; Notenboom, K.; Hekster, Y.A.; Van Den Bemt, B.J.F. The accuracy, precision and sustainability of different techniques for tablet subdivision: Breaking by hand and the use of tablet splitters or a kitchen knife. Int. J. Pharm. 2014, 466, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Ali, G.-C.; Guerchet, M.; Prina, A.M.; Albanese, E.; Wu, Y.-T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer’s Res. Ther. 2016, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasman, A.; Kay, J.; Lieberman, J.A.; First, M.B.; Maj, M. Psychiatry, 3rd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2008. [Google Scholar]
- Small, J.G.; Hirsch, S.R.; Arvanitis, L.A.; Miller, B.G.; Link, C.G.G. Quetiapine in patients with schizophrenia: A high- and low-dose double- blind comparison with placebo. Arch. Gen. Psychiatry 1997, 54, 549–557. [Google Scholar] [CrossRef]
- Worrel, J.A.; Marken, P.A.; Beckman, S.E.; Ruehter, V.L. Atypical antipsychotic agents: A critical review. Am. J. Health Pharm. 2000, 57, 238–255. [Google Scholar] [CrossRef]
- Harding, R.; Peel, E. “He was like a Zombie”: Off-label prescription of antipsychotic drugs in dementia. Med. Law Rev. 2013, 21, 243–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucci, V.; Mandrioli, R.; Ferranti, A.; Furlanetto, S.; Raggi, M.A. Quality control of commercial tablets containing the novel antipsychotic quetiapine. J. Pharm. Biomed. Anal. 2003, 32, 1037–1044. [Google Scholar] [CrossRef]
- Fischbach, M.S.; Gold, J.L.; Lee, M.; Dergal, J.M.; Litner, G.M.; Rochon, P.A. Pill-splitting in a long-term care facility. JAMC 2001, 164, 785–786. [Google Scholar]
- Berg, C.; Ekedahl, A. Dosages involving splitting tablets: Common but unnecessary? J. Pharm. Health Serv. Res. 2010, 1, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Allemann, S.S.; Bornand, D.; Hug, B.; Hersberger, K.E.; Arnet, I. Issues around the Prescription of Half Tablets in Northern Switzerland: The Irrational Case of Quetiapine. Biomed Res. Int. 2015, 2015, 602021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekedahl, B.E.A. Patients′Experiences of Splitting Tablets. Clin. Med. Res. 2013, 2, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Hellberg, A.; Nyängen, L.; Ekedahl, A. Knife, scissors or razor blades…Patients’ experiences and problems with tablets that have to be divided. Lakartidningen 2010, 107, 530–531. [Google Scholar] [PubMed]
- Quinzler, R.; Gasse, C.; Schneider, A.; Kaufmann-Kolle, P.; Szecsenyi, J.; Haefeli, W.E. The frequency of inappropriate tablet splitting in primary care. Eur. J. Clin. Pharmacol. 2006, 62, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines is Publisher. EUROPEAN PHARMACOPOEIA 10 Edition 2.9.5. Uniformity of Mass of Single-Dose Preparations; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2020; p. 335. [Google Scholar]
- European Directorate for the Quality of Medicines is Publisher. EUROPEAN PHARMACOPOEIA 10 Edition 2.9.6 Uniformity of Content of Single-Dose Preparations; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2020; p. 336. [Google Scholar]
- European Directorate for the Quality of Medicines is Publisher. EUROPEAN PHARMACOPOEIA 10 Edition TABLETS Monograph; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2020; p. 937. [Google Scholar]
- Gupta, P.; Gupta, K. Broken tablets: Does the sum of the parts equal the whole? Am. J. Hosp. Pharm. 1988, 45, 1498. [Google Scholar] [CrossRef]
- Sedrati, M.; Arnaud, P.; Fontan, J.E.; Brion, F. Splitting tablets in half. Am. J. Hosp. Pharm. 1994, 51, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Polli, J.E.; Kim, S.; Martin, B.R. Weight uniformity of split tablets required by a Veterans Affairs policy. J. Manag. Care Pharm. 2003, 9, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Geras, D.; Hadziomerovic, D.; Leau, A.; Khan, R.N.; Gudka, S.; Locher, C.; Razaghikashani, M.; Lim, L.Y. Accuracy of tablet splitting and liquid measurements: An examination of who, what and how. J. Pharm. Pharmacol. 2017, 69, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.T.; Sa-Barreto, L.C.L.; Gratieri, T.; Gelfuso, G.M.; Silva, I.C.R.; Cunha-Filho, M.S.S. Key Technical Aspects Influencing the Accuracy of Tablet Subdivision. AAPS PharmSciTech 2017, 18, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Van Der Steen, K.C.; Frijlink, H.W.; Schipper, C.M.A.; Barends, D.M. Prediction of the ease of subdivision of scored tablets from their physical parameters. AAPS PharmSciTech 2010, 11, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verrue, C.; Mehuys, E.; Boussery, K.; Remon, J.P.; Petrovic, M. Tablet-splitting: A common yet not so innocent practice. J. Adv. Nurs. 2011, 67, 26–32. [Google Scholar] [CrossRef]
- Peek, B.T.; Al-Achi, A.; Coombs, S.J. Accuracy of Tablet Splitting by Elderly Patients. J. Am. Med. Assoc. 2002, 399, 451–452. [Google Scholar] [CrossRef]
- Spang, R. Breakability of tablets and film-coated dragees. Pharm. Acta Helv. 1982, 57, 99–111. [Google Scholar] [PubMed]
- Gauthier, S.; Cummings, J.; Ballard, C.; Brodaty, H.; Grossberg, G.; Robert, P.; Lyketsos, C. Management of behavioral problems in Alzheimer’s disease. Int. Psychogeriatr. 2010, 22, 346–372. [Google Scholar] [CrossRef]
- Gustafsson, M.; Karlsson, S.; Lövheim, H. Inappropriate long-term use of antipsychotic drugs is common among people with dementia living in specialized care units. BMC Pharmacol. Toxicol. 2013, 14. [Google Scholar] [CrossRef] [Green Version]
- Trenton, A.F.; Currier, G.W.; Zwemer, F.L. Fatalities associated with therapeutic use and overdose of atypical antipsychotics. CNS Drugs 2003, 17, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Pollak, P.T.; Zbuk, K. Quetiapine fumarate overdose: Clinical and pharmacokinetic lessons from extreme conditions. Clin. Pharmacol. Ther. 2000, 68. [Google Scholar] [CrossRef]
- Bakken, G.V.; Rudberg, I.; Christensen, H.; Molden, E.; Refsum, H.; Hermann, M. Metabolism of quetiapine by CYP3A4 and CYP3A5 in presence or absence of cytochrome B5. Drug Metab. Dispos. 2009, 37, 254–258. [Google Scholar] [CrossRef]
- Wening, K.; Breitkreutz, J. Oral drug delivery in personalized medicine: Unmet needs and novel approaches. Int. J. Pharm. 2011, 404, 1–9. [Google Scholar] [CrossRef]




| Number | Percentage | |
|---|---|---|
| All | 52 | 100 |
| Female | 30 | 57.7 |
| Male | 22 | 42.3 |
| Mean Age ± SD (years) | 85.5 ± 7.2 | - |
| 65–80 | 13 | 25.0 |
| >80 | 39 | 75.0 |
| Diagnosis Of Dementia | ||
| Alzheimer’s | 36 | 69.2 |
| Vascular | 8 | 15.4 |
| Parkinson’s | 4 | 7.7 |
| Front-Temporal | 3 | 5.8 |
| Mixed | 1 | 1.9 |
| Tablets, Quetiapine 100 mg | Tablets, Quetiapine 25 mg | ||||||
|---|---|---|---|---|---|---|---|
| Control | Knife | T. Cutter | Control | Knife | T. Cutter | ||
| Weight comparison | Mean (mg) 1 | 127.0 | 127.3 | 127.5 | 31.5 | 30.8 | 32.1 |
| SD 2 | 1.2 | 15.6 | 19.3 | 0.4 | 7.1 | 6.5 | |
| CV (%) 3 | 0.9 | 12.2 | 15.2 | 1.2 | 23.1 | 20.2 | |
| SEM 4 | 0.3 | 4.5 | 5.6 | 0.1 | 2.1 | 1.9 | |
| Normal distribution 5 | Yes | Yes | Yes | Yes | Yes | Yes | |
| Homoscedasticity 6 | / | Yes | / | Yes | |||
| t-test | / | p-value = 0.977 | / | p-value = 0.633 | |||
| Drug content comparison | Mean (mg) 1 | 51.2 | 51.8 | 52.0 | 13.2 | 12.8 | 13.6 |
| SD 2 | 0.8 | 5.4 | 8.0 | 0.2 | 2.9 | 2.9 | |
| CV (%) 3 | 1.6 | 10.3 | 15.4 | 1.7 | 22.5 | 21.2 | |
| SEM 4 | 0.2 | 1.5 | 2.3 | 0.1 | 0.8 | 0.8 | |
| Normal distribution 5 | Yes | Yes | Yes | Yes | Yes | Yes | |
| Homoscedasticity 6 | / | Yes | / | Yes | |||
| t-test | / | p-value = 0.962 | / | p-value = 0.530 | |||
| Pearson correlation | Correlation coefficient (r) | 0.470 | 0.947 | 0.996 | 0.046 | 0.997 | 0.994 |
| p-value | 0.123 | <0.001 | <0.001 | 0.886 | <0.001 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganzetti, R.; Logrippo, S.; Sestili, M.; Caraffa, A.; Cespi, M.; Pelliccioni, G.; Blasi, P.; Bonacucina, G. Tablet Splitting in Elderly Patients with Dementia: The Case of Quetiapine. Pharmaceutics 2021, 13, 1523. https://doi.org/10.3390/pharmaceutics13091523
Ganzetti R, Logrippo S, Sestili M, Caraffa A, Cespi M, Pelliccioni G, Blasi P, Bonacucina G. Tablet Splitting in Elderly Patients with Dementia: The Case of Quetiapine. Pharmaceutics. 2021; 13(9):1523. https://doi.org/10.3390/pharmaceutics13091523
Chicago/Turabian StyleGanzetti, Roberta, Serena Logrippo, Matteo Sestili, Alessandro Caraffa, Marco Cespi, Giuseppe Pelliccioni, Paolo Blasi, and Giulia Bonacucina. 2021. "Tablet Splitting in Elderly Patients with Dementia: The Case of Quetiapine" Pharmaceutics 13, no. 9: 1523. https://doi.org/10.3390/pharmaceutics13091523
APA StyleGanzetti, R., Logrippo, S., Sestili, M., Caraffa, A., Cespi, M., Pelliccioni, G., Blasi, P., & Bonacucina, G. (2021). Tablet Splitting in Elderly Patients with Dementia: The Case of Quetiapine. Pharmaceutics, 13(9), 1523. https://doi.org/10.3390/pharmaceutics13091523