Spotlight on Calcipotriol/Betamethasone Fixed-Dose Combination in Topical Formulations: Is There Still Room for Innovation?
Abstract
:1. Introduction
2. Skin Barrier Disruption in Psoriasis
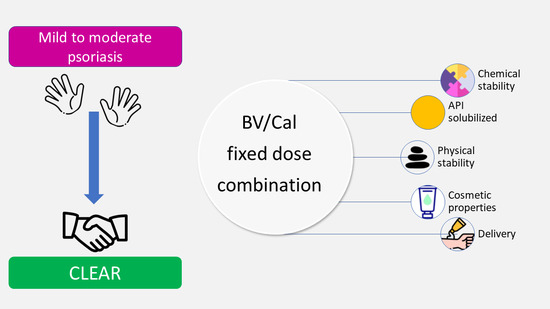
3. Selection of Vehicles in the Design of Fixed-Dose Combination Products
3.1. Topical Vehicles Available on the Market
3.2. Topical Vehicles in R&D
4. Topical Vehicles and Patient’s Preferences
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Byrne, J.; Wyraz, A.; Velasco-Torrijos, T.; Reinhardt, R. Formulation Factors Affecting the Isomerization Rate of Betamethasone-17-Valerate in a Developmental Hydrophilic Cream—A HPLC and Microscopy Based Stability Study. Pharm. Dev. Technol. 2017, 22, 537–544. [Google Scholar] [CrossRef]
- Segaert, S.; Shear, N.H.; Chiricozzi, A.; Thaçi, D.; Carrascosa, J.-M.; Young, H.; Descamps, V. Optimizing Anti-Inflammatory and Immunomodulatory Effects of Corticosteroid and Vitamin D Analogue Fixed-Dose Combination Therapy. Dermatol. Ther. 2017, 7, 265–279. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Benisty, R.; Wormser, U.; Sintov, A.C. Vitamin D3-Based Conjugates for Topical Treatment of Psoriasis: Synthesis, Antiproliferative Activity, and Cutaneous Penetration Studies. Pharm. Res. 2005, 22, 50–57. [Google Scholar] [CrossRef]
- Yip, Y.W.; Po, L.W. The Stability of Betamethasone-17-Valerate in Semi-Solid Bases. J. Pharm. Pharmacol. 1979, 31, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Middleton, K.R.; Haines-Nutt, R.F. The Stability of Diluted Betamethasone 17-Valerate Topical Preparations. In Hospital Pharmacy and the Patient; Springer: Dordrecht, The Netherlands, 1983; pp. 121–126. [Google Scholar] [CrossRef]
- Patel, B.; Siskin, S.; Krazmien, R.; Lebwohl, M. Compatibility of Calcipotriene with Other Topical Medications. J. Am. Acad. Dermatol. 1998, 38, 1010–1011. [Google Scholar] [CrossRef]
- Lebwohl, M. Topical Application of Calcipotriene and Corticosteroids: Combination Regimens. J. Am. Acad. Dermatol. 1997, 37 Pt 2, S55–S58. [Google Scholar] [PubMed]
- Eichenfield, L.F.; Marcoux, D.; Kurvits, M.; Liljedahl, M. Safety and Efficacy of Topical, Fixed-Dose Combination Calcipotriene (0.005%) and Betamethasone (0.064% as Dipropionate) Gel in Adolescent Patients with Scalp and Body Psoriasis: A Phase II Trial. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Beattie, P.E.; Lewis-Jones, M.S. A Comparative Study of Impairment of Quality of Life in Children with Skin Disease and Children with Other Chronic Childhood Diseases. Br. J. Dermatol. 2006, 155, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Segaert, S.; Calzavara-Pinton, P.; de la Cueva, P.; Jalili, A.; Lons Danic, D.; Pink, A.E.; Thaçi, D.; Gooderham, M. Long-Term Topical Management of Psoriasis: The Road Ahead. J. Dermatol. Treat. 2022, 33, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orsmond, A.; Bereza-Malcolm, L.; Lynch, T.; March, L.; Xue, M. Skin Barrier Dysregulation in Psoriasis. Int. J. Mol. Sci. 2021, 22, 10841. [Google Scholar] [CrossRef]
- Takahashi, H.; Tsuji, H.; Minami-Hori, M.; Miyauchi, Y.; Iizuka, H. Defective Barrier Function Accompanied by Structural Changes of Psoriatic Stratum Corneum. J. Dermatol. 2014, 41, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Motta, S.; Monti, M.; Sesana, S.; Caputo, R.; Carelli, S.; Ghidoni, R. Ceramide Composition of the Psoriatic Scale. Biochim. Biophys. Acta-Mol. Basis Dis. 1993, 1182, 147–151. [Google Scholar] [CrossRef]
- van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The Important Role of Stratum Corneum Lipids for the Cutaneous Barrier Function. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2014, 1841, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Opálka, L.; Kováčik, A.; Pullmannová, P.; Maixner, J.; Vávrová, K. Effects of Omega-O-Acylceramide Structures and Concentrations in Healthy and Diseased Skin Barrier Lipid Membrane Models. J. Lipid Res. 2020, 61, 219–228. [Google Scholar] [CrossRef]
- Oestmann, E.; Lavrijsen, A.P.; Hermans, J.; Ponec, M. Skin Barrier Function in Healthy Volunteers as Assessed by Transepidermal Water Loss and Vascular Response to Hexyl Nicotinate: Intra- and Inter-Individual Variability. Br. J. Dermatol. 1993, 128, 130–136. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Yang, S.-H.; Chen, C.-C.; Kao, H.-C.; Fang, J.-Y. Using Imiquimod-Induced Psoriasis-Like Skin as a Model to Measure the Skin Penetration of Anti-Psoriatic Drugs. PLoS ONE 2015, 10, e0137890. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, D.; Horváth, S.; Kemény, Á.; Varga-Medveczky, Z.; Pongor, C.; Molnár, R.; Mihály, A.; Farkas, D.; Naszlady, B.M.; Fülöp, A.; et al. Drug Delivery through the Psoriatic Epidermal Barrier-A “Skin-On-A-Chip” Permeability Study and Ex Vivo Optical Imaging. Int. J. Mol. Sci. 2022, 23, 4237. [Google Scholar] [CrossRef] [PubMed]
- Mok, B.R.; Shon, S.-J.; Kim, A.R.; Simard-Bisson, C.; Martel, I.; Germain, L.; Kim, D.H.; Shin, J.U. Structural and Functional Validation of a Full-Thickness Self-Assembled Skin Equivalent for Disease Modeling. Pharmaceutics 2022, 14, 1211. [Google Scholar] [CrossRef] [PubMed]
- Pasch, M.C. Nail Psoriasis: A Review of Treatment Options. Drugs 2016, 76, 675–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudnicka, L.; Olszewska, M.; Goldust, M.; Waśkiel-Burnat, A.; Warszawik-Hendzel, O.; Dorożyński, P.; Turło, J.; Rakowska, A. Efficacy and Safety of Different Formulations of Calcipotriol/Betamethasone Dipropionate in Psoriasis: Gel, Foam, and Ointment. J. Clin. Med. 2021, 10, 5589. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.; Høy, G.; Didriksen, E.; Persson, J.; Melchior, N.; Hansen, J. Development of a New Formulation Combining Calcipotriol and Betamethasone Dipropionate in an Ointment Vehicle. Drug Dev. Ind. Pharm. 2004, 30, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L. Calcipotriol/Betamethasone Dipropionate: A Review of Its Use in the Treatment of Psoriasis Vulgaris of the Trunk, Limbs and Scalp. Drugs 2011, 71, 709–730. [Google Scholar] [CrossRef] [PubMed]
- Dumas, K.J.; Scholtz, J.R. The Psoriasis Bio-Assay for Topical Corticosteroid Activity. Acta Derm. Venereol. 1972, 52, 43–48. [Google Scholar] [PubMed]
- Queille-Roussel, C.; Hoffmann, V.; Enevold, A.; Ganslandt, C. Use of a Psoriasis Plaque Test in the Development of a Gel Formulation of Calcipotriol and Betamethasone Dipropionate for Scalp Psoriasis. J. Dermatol. Treat. 2013, 24, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Gennari, C.G.M.; Selmin, F.; Minghetti, P.; Cilurzo, F. Medicated Foams and Film Forming Dosage Forms as Tools to Improve the Thermodynamic Activity of Drugs to Be Administered through the Skin. Curr. Drug Deliv. 2019, 16, 461–471. [Google Scholar] [CrossRef]
- Lind, M.; Nielsen, K.T.; Schefe, L.H.; Nørremark, K.; Eriksson, A.H.; Norsgaard, H.; Pedersen, B.T.; Petersson, K. Supersaturation of Calcipotriene and Betamethasone Dipropionate in a Novel Aerosol Foam Formulation for Topical Treatment of Psoriasis Provides Enhanced Bioavailability of the Active Ingredients. Dermatol. Ther. 2016, 6, 413–425. [Google Scholar] [CrossRef]
- Sebba, F. Biliquid Foams—A Preliminary Report. J. Colloid Interface Sci. 1972, 40, 468–474. [Google Scholar] [CrossRef]
- Molaei, A.; Waters, K.E. Aphron Applications—A Review of Recent and Current Research. Adv. Colloid Interface Sci. 2015, 216, 36–54. [Google Scholar] [CrossRef]
- Moiseev, R.V.; Steele, F.; Khutoryanskiy, V.V. Polyaphron Formulations Stabilised with Different Water-Soluble Polymers for Ocular Drug Delivery. Pharmaceutics 2022, 14, 926. [Google Scholar] [CrossRef]
- Praestegaard, M.; Steele, F.; Crutchley, N. Polyaphron Dispersion Technology, A Novel Topical Formulation and Delivery System Combining Drug Penetration, Local Tolerability and Convenience of Application. Dermatol. Ther. 2022, 12, 2217–2231. [Google Scholar] [CrossRef]
- Praestegaard, N.; Crutchley, M.; Georgiou, S.; Morten, L. Topical Composition Comprising Calcipotriol and Betamethasone Dipropionate. Patent EP3542788A1, 25 September 2019. [Google Scholar]
- Wheeler, D.; Steele, D.F.; Georgiou, M.; Sindet-Pedersen, S. Topical Compositio. U.S. Patent US11065195B2, 23 April 2019. [Google Scholar]
- Lallemand, F.; Schmitt, M.; Bourges, J.; Gurny, R.; Benita, S.; Garrigue, J. Cyclosporine A Delivery to the Eye: A Comprehensive Review of Academic and Industrial Efforts. Eur. J. Pharm. Biopharm. 2017, 117, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Habjanič, N.; Kerec Kos, M.; Kristan, K. Sensitivity of Different In Vitro Performance Tests and Their In Vivo Relevance for Calcipotriol/Betamethasone Ointment. Pharm. Res. 2020, 37, 52. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.F.S. Topical Therapies for Psoriasis: Improving Management Strategies and Patient Adherence. Semin. Cutan. Med. Surg. 2016, 35, S36–S46. [Google Scholar] [CrossRef]
- Liang, L.; Fei, W.M.; Zhao, Z.Q.; Hao, Y.Y.; Zhang, C.; Cui, Y.; Guo, X.D. Improved Imiquimod-Induced Psoriasis like Dermatitis Using Microneedles in Mice. Eur. J. Pharm. Biopharm. 2021, 164, 20–27. [Google Scholar] [CrossRef]
- Microneedle Patch for Psoriatic Plaques. ClinicalTrials.gov Identifier: NCT02955576. Available online: https://clinicaltrials.gov/ct2/show/NCT02955576 (accessed on 4 August 2022).
- Lee, J.H.; Jung, Y.S.; Kim, G.M.; Bae, J.M. A Hyaluronic Acid-Based Microneedle Patch to Treat Psoriatic Plaques: A Pilot Open Trial. Br. J. Dermatol. 2018, 178, e24–e25. [Google Scholar] [CrossRef]
- Sonawane, R.; Harde, H.; Katariya, M.; Agrawal, S.; Jain, S. Solid Lipid Nanoparticles-Loaded Topical Gel Containing Combination Drugs: An Approach to Offset Psoriasis. Expert Opin. Drug Deliv. 2014, 11, 1833–1847. [Google Scholar] [CrossRef]
- Bhat, M.; Pukale, S.; Singh, S.; Mittal, A.; Chitkara, D. Nano-Enabled Topical Delivery of Anti-Psoriatic Small Molecules. J. Drug Deliv. Sci. Technol. 2021, 62, 102328. [Google Scholar] [CrossRef]
- Saleem, S.; Iqubal, M.K.; Garg, S.; Ali, J.; Baboota, S. Trends in Nanotechnology-Based Delivery Systems for Dermal Targeting of Drugs: An Enticing Approach to Offset Psoriasis. Expert Opin. Drug Deliv. 2020, 17, 817–838. [Google Scholar] [CrossRef]
- Kumar, R.; Dogra, S.; Amarji, B.; Singh, B.; Kumar, S.; Sharma, S.; Vinay, K.; Mahajan, R.; Katare, O.P. Efficacy of Novel Topical Liposomal Formulation of Cyclosporine in Mild to Moderate Stable Plaque Psoriasis: A Randomized Clinical Trial. JAMA Dermatol. 2016, 152, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, N.Ø.; Rønholt, S.; Salte, R.D.; Jorgensen, L.; Thormann, T.; Basse, L.H.; Hansen, J.; Frokjaer, S.; Foged, C. Calcipotriol Delivery into the Skin with PEGylated Liposomes. Eur. J. Pharm. Biopharm. 2012, 81, 532–539. [Google Scholar] [CrossRef]
- Chandra, A.; Aggarwal, G.; Manchanda, S.; Narula, A. Development of Topical Gel of Methotrexate Incorporated Ethosomes and Salicylic Acid for the Treatment of Psoriasis. Pharm. Nanotechnol. 2019, 7, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Katiyar, S.S.; Kushwah, V.; Jain, S. Nanoemulsion Loaded Gel for Topical Co-Delivery of Clobitasol Propionate and Calcipotriol in Psoriasis. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Katiyar, S.S.; Kushwah, V.; Jain, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carrier-Based Nanotherapeutics in Treatment of Psoriasis: A Comparative Study. Expert Opin. Drug Deliv. 2017, 14, 165–177. [Google Scholar] [CrossRef]
- Kardas, P.; Lewek, P.; Matyjaszczyk, M. Determinants of Patient Adherence: A Review of Systematic Reviews. Front. Pharmacol. 2013, 4, 91. [Google Scholar] [CrossRef] [PubMed]
- Rapp, S.R.; Exum, M.L.; Reboussin, D.M.; Feldman, S.R.; Fleischer, A.; Clark, A. The Physical, Psychological and Social Impact of Psoriasis. J. Health Psychol. 1997, 2, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Richards, H.L.; Fortune, D.G.; O’Sullivan, T.M.; Main, C.J.; Griffiths, C.E.M. Patients with Psoriasis and Their Compliance with Medication. J. Am. Acad. Dermatol. 1999, 41, 581–583. [Google Scholar] [CrossRef]
- Müller, S.M.; Tomaschett, D.; Euler, S.; Vogt, D.R.; Herzog, L.; Itin, P. Topical Corticosteroid Concerns in Dermatological Outpatients: A Cross-Sectional and Interventional Study. Dermatology 2016, 232, 444–452. [Google Scholar] [CrossRef]
- Feldman, S.R.; Horn, E.J.; Balkrishnan, R.; Basra, M.K.; Finlay, A.Y.; McCoy, D.; Menter, A.; van de Kerkhof, P.C.M. Psoriasis: Improving Adherence to Topical Therapy. J. Am. Acad. Dermatol. 2008, 59, 1009–1016. [Google Scholar] [CrossRef]
- Menditto, E.; Orlando, V.; De Rosa, G.; Minghetti, P.; Musazzi, U.M.; Cahir, C.; Kurczewska-Michalak, M.; Kardas, P.; Costa, E.; Sousa Lobo, J.M.; et al. Patient Centric Pharmaceutical Drug Product Design-The Impact on Medication Adherence. Pharmaceutics 2020, 12, 44. [Google Scholar] [CrossRef] [Green Version]
- Svendsen, M.T.; Feldman, S.R.; Tiedemann, S.N.; Sørensen, A.S.S.; Rivas, C.M.R.; Andersen, K.E. Psoriasis Patient Preferences for Topical Drugs: A Systematic Review. J. Dermatol. Treat. 2021, 32, 478–483. [Google Scholar] [CrossRef]
- Ivens, U.I.; Steinkjer, B.; Serup, J.; Tetens, V. Ointment Is Evenly Spread on the Skin, in Contrast to Creams and Solutions. Br. J. Dermatol. 2001, 145, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Vasconcelos, V.; Teixeira, M.; Almeida, V.; Azevedo, R.; Torres, T.; Sousa Lobo, J.M.; Costa, P.C.; Almeida, I.F. Mechanical Properties of Topical Anti-Psoriatic Medicines: Implications for Patient Satisfaction with Treatment. AAPS PharmSciTech. 2019, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Felix, K.; Unrue, E.; Inyang, M.; Cardwell, L.A.; Oussedik, E.; Richardson, I.; Feldman, S.R. Patients Preferences for Different Corticosteroid Vehicles Are Highly Variable. J. Dermatol. Treat. 2020, 31, 147–151. [Google Scholar] [CrossRef]
- Reich, A.; Selmer, J.; Galván, J.; Trebbien, P.; Pi-Blanque, A.; Danø, A.; Stallknecht, S.E.; Bewley, A. Efficacy, Quality of Life, and Treatment Satisfaction: An Indirect Comparison of Calcipotriol/Betamethasone Dipropionate Cream versus Foam for Treatment of Psoriasis. Curr. Med. Res. Opin. 2022, 38, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.A.; Thoning, H.; Gerdes, S.; Megna, M.; Brandi, H.; Bernasconi, M.Y.J.; Yélamos, O. Matching-Adjusted Indirect Comparison of Efficacy Outcomes in Trials of Calcipotriol plus Betamethasone Dipropionate Foam and Cream Formulations for the Treatment of Plaque Psoriasis. J. Dermatol. Treat. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.R.; Levi, E.; Pathak, P.; Kakatkar, S.; Balkrishnan, R. First-Line Fixed-Combination Psoriasis Treatment Is Associated With Lower Healthcare Costs. Skinmed 2016, 14, 266–272. [Google Scholar] [PubMed]


| Formulation | Trade Name | Skin Source | Flux (ng/cm2 h−1) | Reference | |
|---|---|---|---|---|---|
| BD | Cal | ||||
| Ointment | Daivonex® | Pig | NA * | 6.7 ± 0.4 | [22] |
| Diproderm® | 50 ± 9 | NA * | |||
| Ointment | Generic product | Human | 2.81 ± 0.28 | 0.23 ± 0.04 | [35] |
| Daivobet® | 1.93 ± 0.29 | 0.13 ± 0.05 | |||
| FoamOintment | Enstillar® | Pig | -- * | 0.005 | Estimated from [27] |
| -- | -- * | 0.002 | |||
| Oleogel/suspensionCream | -- | Human | ~34 | ~4 | [31] |
| Wynzora® | ~68 | ~12 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selmin, F.; Franzè, S.; Casiraghi, A.; Cilurzo, F. Spotlight on Calcipotriol/Betamethasone Fixed-Dose Combination in Topical Formulations: Is There Still Room for Innovation? Pharmaceutics 2022, 14, 2085. https://doi.org/10.3390/pharmaceutics14102085
Selmin F, Franzè S, Casiraghi A, Cilurzo F. Spotlight on Calcipotriol/Betamethasone Fixed-Dose Combination in Topical Formulations: Is There Still Room for Innovation? Pharmaceutics. 2022; 14(10):2085. https://doi.org/10.3390/pharmaceutics14102085
Chicago/Turabian StyleSelmin, Francesca, Silvia Franzè, Antonella Casiraghi, and Francesco Cilurzo. 2022. "Spotlight on Calcipotriol/Betamethasone Fixed-Dose Combination in Topical Formulations: Is There Still Room for Innovation?" Pharmaceutics 14, no. 10: 2085. https://doi.org/10.3390/pharmaceutics14102085
APA StyleSelmin, F., Franzè, S., Casiraghi, A., & Cilurzo, F. (2022). Spotlight on Calcipotriol/Betamethasone Fixed-Dose Combination in Topical Formulations: Is There Still Room for Innovation? Pharmaceutics, 14(10), 2085. https://doi.org/10.3390/pharmaceutics14102085