Design of Liposomal Lidocaine/Cannabidiol Fixed Combinations for Local Neuropathic Pain Treatment
Abstract
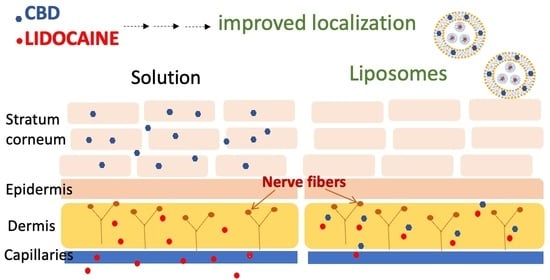
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Differential Scanning Calorimetry (DSC) Studies
2.2.2. Preparation of Liposomes
2.2.3. Physico-Chemical Characterization of Liposomes
2.2.4. In Vitro Drug Release Studies
2.2.5. In Vitro Skin Permeability Studies
3. Results and Discussion
3.1. Physico-Chemical Characterization of Liposomes
3.2. In Vitro Drug Release and Skin Permeability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bates, D.; Schultheis, B.C.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019, 20, S2–S12. [Google Scholar] [CrossRef] [PubMed]
- Sawynok, J. Topical analgesics for neuropathic pain: Preclinical exploration, clinical validation, future development. Eur. J. Pain 2014, 18, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shu, G.F.; Lu, K.J.; Xu, X.L.; Sun, M.C.; Qi, J.; Huang, Q.-L.; Tan, W.-Q.; Du, Y.-Z. Flexible liposomal gel dual-loaded with all-trans retinoic acid and betamethasone for enhanced therapeutic efficiency of psoriasis. J. Nanobiotechnol. 2020, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chen, J.; Feng, S.; Li, M.; Sun, Y.; Liu, Y. Combination anesthetic therapy: Co-delivery of ropivacaine and meloxicam using transcriptional transactivator peptide modified nanostructured lipid carriers in vitro and in vivo. Drug Deliv. 2022, 29, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Suo, M.; Zhao, X.; Yu, G.; Zhang, W. Lidocaine loaded nanostructured lipid carriers for prolonged local anesthesia: In vitro and in vivo studies. J. Dispers. Sci. Technol. 2022, 43, 682–689. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, H.; Zou, Z. In Vitro and in Vivo Evaluation of a Novel Lidocaine-Loaded Cubosomal Gel for Prolonged Local Anesthesia. J. Biomater. Appl. 2022, 37, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, A.; Musazzi, U.M.; Centin, G.; Franzè, S.; Minghetti, P. Topical Administration of Cannabidiol: Influence of Vehicle-Related Aspects on Skin Permeation Process. Pharmaceuticals 2020, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Lodzki, M.; Godin, B.; Rakou, L.; Mechoulam, R.; Gallily, R.; Touitou, E. Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. J. Control Release 2003, 93, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Franzè, S.; Musazzi, U.M.; Minghetti, P.; Cilurzo, F. Drug-in-micelles-in-liposomes (DiMiL) systems as a novel approach to prevent drug leakage from deformable liposomes. Eur. J. Pharm. Sci. 2019, 130, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Franzé, S.; Marengo, A.; Stella, B.; Minghetti, P.; Arpicco, S.; Cilurzo, F. Hyaluronan-decorated liposomes as drug delivery systems for cutaneous administration. Int. J. Pharm. 2018, 535, 333–339. [Google Scholar] [CrossRef]
- Franzè, S.; Donadoni, G.; Podestà, A.; Procacci, P.; Orioli, M.; Carini, M.; Minghetti, P.; Cilurzo, F. Tuning the extent and the depth of penetration of flexible liposomes in human skin. Mol. Pharm. 2017, 14, 1998–2009. [Google Scholar] [CrossRef]
- Fatima, M.T.; Islam, Z.; Ahmad, E.; Barreto, G.E.; Md Ashraf, G. Ionic gradient liposomes: Recent advances in the stable entrapment and prolonged released of local anesthetics and anticancer drugs. Biomed. Pharmacother. 2018, 107, 34–43. [Google Scholar] [CrossRef]
- Omar, M.M.; Hasan, O.A.; El Sisi, A.M. Preparation and optimization of lidocaine transferosomal gel containing permeation enhancers: A promising approach for enhancement of skin permeation. Int. J. Nanomed. 2019, 14, 1551–1562. [Google Scholar] [CrossRef]
- Carafa, M.; Santucci, E.; Lucania, G. Lidocaine-loaded non-ionic surfactant vesicles: Characterization and in vitro permeation studies. Int. J. Pharm. 2002, 231, 21–32. [Google Scholar] [CrossRef]
- Junaid, M.S.A.; Tijani, A.O.; Puri, A.; Banga, A.K. In vitro percutaneous absorption studies of cannabidiol using human skin: Exploring the effect of drug concentration, chemical enhancers, and essential oils. Int. J. Pharm. 2022, 616, 121540. [Google Scholar] [CrossRef]




| Form. | Lipid Bilayer | Aqueous Phase | Physico-Chemical Characteristics | |||||
|---|---|---|---|---|---|---|---|---|
| Tween 80 (% w/w) | sPC (% w/w) | Inner Core | Dispersant Medium | EE% | d (nm) | Deformability K (N/mm) | ||
| CBD | LD | |||||||
| DiMiL | 15 | 85 | K-HS15 5 w/v% | Depurated water | 92.2 ± 3.4 | 59.3 ± 0.3 | 81.3 ± 0.2 | 0.001 ± 0.000 |
| DiMiL(-) | - | 100 | K-HS15 5 w/v% | Depurated water | 94.0 ± 5.0 | 74.8 ± 4.6 | 96.0 ± 0.6 | 0.018 ± 0.007 |
| G* | 15 | 85 | Buffer pH 2.5 | Buffer pH 6.5 | 63.7 ± 4.1 | 53.9 ± 3.0 | 107.1 ± 0.7 | 0.003 ± 0.001 |
| G(-) | - | 100 | Buffer pH 2.5 | Buffer pH 6.5 | 76.0 ± 1.4 | 88.9 ± 2.5 | 103.0 ± 0.2 | 0.040 ± 0.009 |
| Formulation | Steady-State Flux (μg/cm2/h) | |
|---|---|---|
| LD | CBD | |
| Versatis lidocaine plaster * | 7.51 ± 1.27 | - |
| 1% CBD PEG 400 solution ** | - | 0.03 ± 0.01 |
| G(-) | 0.35 ± 0.06 | 0.07 ± 0.02 |
| G | 0.28 ± 0.16 | 0.12 ± 0.03 |
| DIMIL(-) | 3.00 ± 0.82 | 0.19 ± 0.10 |
| DIMIL | 5.62 ± 2.42 | 0.10 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzè, S.; Angelo, L.; Casiraghi, A.; Minghetti, P.; Cilurzo, F. Design of Liposomal Lidocaine/Cannabidiol Fixed Combinations for Local Neuropathic Pain Treatment. Pharmaceutics 2022, 14, 1915. https://doi.org/10.3390/pharmaceutics14091915
Franzè S, Angelo L, Casiraghi A, Minghetti P, Cilurzo F. Design of Liposomal Lidocaine/Cannabidiol Fixed Combinations for Local Neuropathic Pain Treatment. Pharmaceutics. 2022; 14(9):1915. https://doi.org/10.3390/pharmaceutics14091915
Chicago/Turabian StyleFranzè, Silvia, Liliana Angelo, Antonella Casiraghi, Paola Minghetti, and Francesco Cilurzo. 2022. "Design of Liposomal Lidocaine/Cannabidiol Fixed Combinations for Local Neuropathic Pain Treatment" Pharmaceutics 14, no. 9: 1915. https://doi.org/10.3390/pharmaceutics14091915
APA StyleFranzè, S., Angelo, L., Casiraghi, A., Minghetti, P., & Cilurzo, F. (2022). Design of Liposomal Lidocaine/Cannabidiol Fixed Combinations for Local Neuropathic Pain Treatment. Pharmaceutics, 14(9), 1915. https://doi.org/10.3390/pharmaceutics14091915