Dermal Delivery of Diclofenac Sodium—In Vitro and In Vivo Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. HPLC Analysis
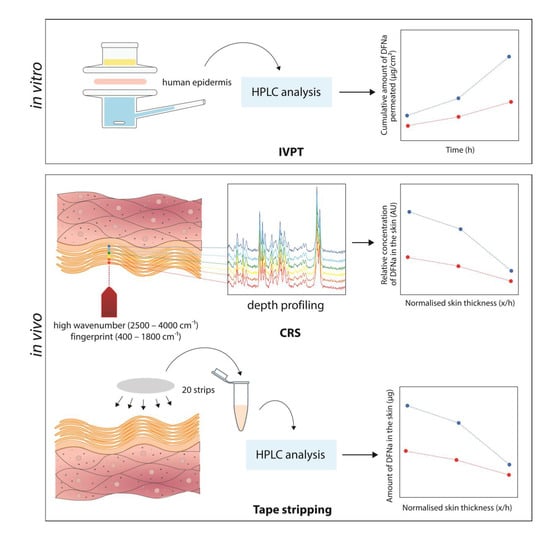
2.2.2. In Vitro Permeation Test (IVPT) Studies
2.2.3. Confocal Raman Spectroscopy
2.2.4. Tape Stripping
2.2.5. Data Analysis
3. Results and Discussion
3.1. In Vitro Permeation Studies
3.2. In Vivo Studies
3.2.1. Confocal Raman Spectroscopy
3.2.2. Tape Stripping
3.3. In Vitro–In Vivo Correlations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, P.; Raney, S.; Luke, M. Evaluation of Cutaneous Pharmacokinetics: The Past, the Present and the Future; SPIE: Bellingham, DC, USA, 2021; Volume 11624. [Google Scholar]
- Raney, S.G.; Franz, T.J.; Lehman, P.A.; Lionberger, R.; Chen, M.-L. Pharmacokinetics-Based Approaches for Bioequivalence Evaluation of Topical Dermatological Drug Products. Clin. Pharmacokinet. 2015, 54, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Kezic, S. Methods for measuring in-vivo percutaneous absorption in humans. Hum. Exp. Toxicol. 2008, 27, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Lehman, P.A.; Raney, S.G.; Franz, T.J. Percutaneous Absorption in Man: In vitro-in vivo Correlation. Skin Pharmacol. Physiol. 2011, 24, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, D.; Rougier, A.; Roguet, R.; Lotte, C.; Kalopissis, G. In vivo Relationship between Horny Layer Reservoir Effect and Percutaneous Absorption in Human and Rat. J. Investig. Dermatol. 1984, 82, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration In Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef] [Green Version]
- Keurentjes, A.J.; Jakasa, I.; Kezic, S. Research Techniques Made Simple: Stratum Corneum Tape Stripping. J. Investig. Dermatol. 2021, 141, 1129–1133.e1121. [Google Scholar] [CrossRef]
- Bashir, S.J.; Chew, A.L.; Anigbogu, A.; Dreher, F.; Maibach, H.I. Physical and physiological effects of stratum corneum tape stripping. Ski. Res. Technol. 2001, 7, 40–48. [Google Scholar] [CrossRef]
- Jacobi, U.; Meykadeh, N.; Sterry, W.; Lademann, J. Effect of the vehicle on the amount of stratum corneum removed by tape stripping. JDDG J. Dtsch. Dermatol. Ges. 2003, 1, 884–889. [Google Scholar] [CrossRef]
- Löffler, H.; Dreher, F.; Maibach, H.I. Stratum corneum adhesive tape stripping: Influence of anatomical site, application pressure, duration and removal. Br. J. Dermatol. 2004, 151, 746–752. [Google Scholar] [CrossRef]
- Lademann, J.; Jacobi, U.; Surber, C.; Weigmann, H.J.; Fluhr, J.W. The tape stripping procedure—Evaluation of some critical parameters. Eur. J. Pharm. Biopharm. 2009, 72, 317–323. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Draft Guidance for Industry on Topical Dermatological Drug Product NDAs and ANDAs-In Vivo Bioavailability, Bioequivalence, In Vitro Release and Associated Studies; Withdrawal. Fed. Regist. 2002, 67, 35122–35123. [Google Scholar]
- Goh, C.F.; Moffat, J.G.; Craig, D.Q.M.; Hadgraft, J.; Lane, M.E. Monitoring Drug Crystallization in Percutaneous Penetration Using Localized Nanothermal Analysis and Photothermal Microspectroscopy. Mol. Pharm. 2019, 16, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Hadgraft, J.; Lane, M.E. Drug crystallization—Implications for topical and transdermal delivery. Expert Opin. Drug Deliv. 2016, 13, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Caspers, P.J.; Lucassen, G.W.; Wolthuis, R.; Bruining, H.A.; Puppels, G.J. In vitro and in vivo Raman spectroscopy of human skin. Biospectroscopy 1998, 4, S31–S39. [Google Scholar] [CrossRef]
- Caspers, P.J.; Bruining, H.A.; Puppels, G.J.; Lucassen, G.W.; Carter, E.A. In Vivo Confocal Raman Microspectroscopy of the Skin: Noninvasive Determination of Molecular Concentration Profiles. J. Investig. Dermatol. 2001, 116, 434–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieber, C.; Mahadevan-Jansen, A. Development of a handheld Raman microspectrometer for clinical dermatologic applications. Opt. Express 2007, 15, 11874–11882. [Google Scholar] [CrossRef] [PubMed]
- Franzen, L.; Windbergs, M. Applications of Raman spectroscopy in skin research—From skin physiology and diagnosis up to risk assessment and dermal drug delivery. Adv. Drug Deliv. Rev. 2015, 89, 91–104. [Google Scholar] [CrossRef]
- van der Pol, A.; Caspers, P.J. Confocal Raman Spectroscopy for In Vivo Skin Hydration Measurement. In Handbook of Cosmetic Science and Technology; CRC Press: Boca Raton, FL, USA, 2019; pp. 169–182. [Google Scholar]
- Pena, A.-M.; Chen, X.; Pence, I.J.; Bornschlögl, T.; Jeong, S.; Grégoire, S.; Luengo, G.S.; Hallegot, P.; Obeidy, P.; Feizpour, A.; et al. Imaging and quantifying drug delivery in skin—Part 2: Fluorescence andvibrational spectroscopic imaging methods. Adv. Drug Deliv. Rev. 2020, 153, 147–168. [Google Scholar] [CrossRef]
- Pudney, P.D.A.; Mélot, M.; Caspers, P.J.; van der Pol, A.; Puppels, G.J. An In Vivo Confocal Raman Study of the Delivery of Trans-Retinol to the Skin. Appl. Spectrosc. 2007, 61, 804–811. [Google Scholar] [CrossRef]
- Mélot, M.; Pudney, P.D.A.; Williamson, A.-M.; Caspers, P.J.; Van Der Pol, A.; Puppels, G.J. Studying the effectiveness of penetration enhancers to deliver retinol through the stratum cornum by in vivo confocal Raman spectroscopy. J. Control Release 2009, 138, 32–39. [Google Scholar] [CrossRef]
- Mateus, R.; Abdalghafor, H.; Oliveira, G.; Hadgraft, J.; Lane, M.E. A new paradigm in dermatopharmacokinetics—Confocal Raman spectroscopy. Int. J. Pharm. 2013, 444, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Pig Ear Skin ex Vivo as a Model for in Vivo Dermatopharmacokinetic Studies in Man. Pharm. Res. 2006, 23, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Enhanced permeation of fentanyl from supersaturated solutions in a model membrane. Int. J. Pharm. 2011, 407, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Mateus, R.; Moore, D.J.; Hadgraft, J.; Lane, M.E. Percutaneous absorption of salicylic acid—In vitro and in vivo studies. Int. J. Pharm. 2014, 475, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In Vitro–In Vivo Correlation in Skin Permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, F.; Caspers, P.J.; Puppels, G.J.; Lane, M.E. Franz Cell Diffusion Testing and Quantitative Confocal Raman Spectroscopy: In Vitro-In Vivo Correlation. Pharmaceutics 2020, 12, 887. [Google Scholar] [CrossRef]
- Skelly, J.P.; Shah, V.P.; Maibach, H.I.; Guy, R.H.; Wester, R.C.; Flynn, G.; Yacobi, A. FDA and AAPS Report of the Workshop on Principles and Practices of In Vitro Percutaneous Penetration Studies: Relevance to Bioavailability and Bioequivalence. Pharm. Res. 1987, 4, 265–267. [Google Scholar] [CrossRef]
- Franz, T.J.; Lehman, P.A.; Raney, S.G. Use of Excised Human Skin to Assess the Bioequivalence of Topical Products. Skin Pharmacol. Physiol. 2009, 22, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.H.; Thomas, S.; Raney, S.G.; Ghosh, P.; Hammell, D.C.; El-Kamary, S.S.; Chen, W.H.; Billington, M.M.; Hassan, H.E.; Stinchcomb, A.L. In vitro–in vivo correlations for nicotine transdermal delivery systems evaluated by both in vitro skin permeation (IVPT) and in vivo serum pharmacokinetics under the influence of transient heat application. J. Control Release 2018, 270, 76–88. [Google Scholar] [CrossRef]
- Shin, S.H.; Rantou, E.; Raney, S.G.; Ghosh, P.; Hassan, H.; Stinchcomb, A. Cutaneous Pharmacokinetics of Acyclovir Cream 5% Products: Evaluating Bioequivalence with an In Vitro Permeation Test and an Adaptation of Scaled Average Bioequivalence. Pharm. Res. 2020, 37, 210. [Google Scholar] [CrossRef]
- Pyatski, Y.; Zhang, Q.; Mendelsohn, R.; Flach, C.R. Effects of permeation enhancers on flufenamic acid delivery in Ex vivo human skin by confocal Raman microscopy. Int. J. Pharm. 2016, 505, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.F.; Lane, M.E. Formulation of diclofenac for dermal delivery. Int. J. Pharm. 2014, 473, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.J.; Herranz, P.; Cruz, S.B.; Parodi, A. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: Potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol. Ther. 2019, 32, e12800. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.F.; Boyd, B.J.; Craig, D.Q.M.; Lane, M.E. Profiling of drug crystallization in the skin. Expert Opin. Drug Deliv. 2020, 17, 1321–1334. [Google Scholar] [CrossRef]
- Goh, C.F.; Craig, D.Q.M.; Hadgraft, J.; Lane, M.E. The application of ATR-FTIR spectroscopy and multivariate data analysis to study drug crystallisation in the stratum corneum. Eur. J. Pharm. Biopharm. 2017, 111, 16–25. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite. Validation of analytical procedures: Text and methodology Q2 (R1). In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 6 November 2005. [Google Scholar]
- Organization for Economic Cooperation Development. Test No. 428: Skin absorption: In vitro method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation Development. OECD Guidance document for the conduct of skin absorption studies. In OECD Series on Testing and Assessment; OECD: Paris, France, 2004. [Google Scholar]
- Iliopoulos, F.; Chapman, A.; Lane, M.E. A comparison of the in vitro permeation of 3-O-ethyl-l-ascorbic acid in human skin and in a living skin equivalent (LabSkin™). Int. J. Cosmet. Sci. 2021, 43, 107–112. [Google Scholar] [CrossRef]
- Oliveira, G.; Hadgraft, J.; Lane, M.E. The influence of volatile solvents on transport across model membranes and human skin. Int. J. Pharm. 2012, 435, 38–49. [Google Scholar] [CrossRef]
- The RefData Foundation. Primofenac® Emulsions-Gel Patient Information. Medicinal Product Information Search Platform (AIPS) for Authorised Human Medicines. 2020. Available online: https://www.swissmedicinfo.ch/ (accessed on 8 June 2022).
- Patel, A.; Iliopoulos, F.; Caspers, P.J.; Puppels, G.J.; Lane, M.E. In Vitro–In Vivo Correlation in Dermal Delivery: The Role of Excipients. Pharmaceutics 2021, 13, 542. [Google Scholar] [CrossRef]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. Depth profiling of stratum corneum biophysical and molecular properties. Br. J. Dermatol. 2011, 164, 957–965. [Google Scholar] [CrossRef]
- Zhang, Y.; Kung, C.-P.; Iliopoulos, F.; Sil, B.C.; Hadgraft, J.; Lane, M.E. Dermal Delivery of Niacinamide—In Vivo Studies. Pharmaceutics 2021, 13, 726. [Google Scholar] [CrossRef]
- Caspers, P.; Lucassen, G.; Bruining, H.; Puppels, G. Automated depth-scanning confocal Raman microspectrometer for rapid in vivo determination of water concentration profiles in human skin. J. Raman Spectrosc. 2000, 31, 813–818. [Google Scholar] [CrossRef]
- Crowther, J.M.; Sieg, A.; Blenkiron, P.; Marcott, C.; Matts, P.J.; Kaczvinsky, J.R.; Rawlings, A.V. Measuring the effects of topical moisturizers on changes in stratum corneum thickness, water gradients and hydration in vivo. Br. J. Dermatol. (1951) 2008, 159, 567–577. [Google Scholar] [CrossRef]
- Kalia, Y.N.; Alberti, I.; Naik, A.; Guy, R.H. Assessment of Topical Bioavailability in vivo: The Importance of Stratum corneum Thickness. Skin Pharmacol. Physiol. 2001, 14 (Suppl 1), 82–86. [Google Scholar] [CrossRef] [PubMed]
- Voegeli, R.; Heiland, J.; Doppler, S.; Rawlings, A.V.; Schreier, T. Efficient and simple quantification of stratum corneum proteins on tape strippings by infrared densitometry. Skin Res. Technol. 2007, 13, 242–251. [Google Scholar] [CrossRef]
- Mohammed, D.; Yang, Q.; Guy, R.H.; Matts, P.J.; Hadgraft, J.; Lane, M.E. Comparison of gravimetric and spectroscopic approaches to quantify stratum corneum removed by tape-stripping. Eur. J. Pharm. Biopharm. 2012, 82, 171–174. [Google Scholar] [CrossRef]
- Team, R. Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2021. [Google Scholar]
- Finnin, B.; Walters, K.A.; Franz, T.J. In vitro skin permeation methodology. In Transdermal and Topical Drug Delivery: Principles and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 85–108. [Google Scholar]
- Loth, H. Vehicular influence on transdermal drug penetration. Int. J. Pharm. 1991, 68, 1–10. [Google Scholar] [CrossRef]
- Kakubari, I.; Sasaki, H.; Takayasu, T.; Yamauchi, H.; Takayama, S.; Takayama, K. Effects of ethylcellulose and 2-octyldodecanol additives on skin permeation and irritation with ethylene-vinyl acetate copolymer matrix patches containing formoterol fumarate. Biol. Pharm. Bull. 2006, 29, 1717–1722. [Google Scholar] [CrossRef] [Green Version]
- Ameen, D.; Michniak-Kohn, B. Development and in vitro evaluation of pressure sensitive adhesive patch for the transdermal delivery of galantamine: Effect of penetration enhancers and crystallization inhibition. Eur. J. Pharm. Biopharm. 2019, 139, 262–271. [Google Scholar] [CrossRef]
- Montenegro, L.; Carbone, C.; Puglisi, G. Vehicle effects on in vitro release and skin permeation of octylmethoxycinnamate from microemulsions. Int. J. Pharm. 2011, 405, 162–168. [Google Scholar] [CrossRef]
- Mitriaikina, S.; Muller-Goymann, C.C. Synergetic effects of isopropyl alcohol (IPA) and isopropyl myristate (IPM) on the permeation of betamethasone-17-valerate from semisolid Pharmacopoeia bases. J. Drug Deliv. Sci. Technol. 2007, 17, 1–8. [Google Scholar] [CrossRef]
- Lane, M.E.; Santos, P.; Watkinson, A.C.; Hadgraft, J. Passive Skin Permeation Enhancement. In Topical and Transdermal Drug Delivery; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 23–42. [Google Scholar] [CrossRef]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, F.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. 3-O-ethyl-l-ascorbic acid: Characterisation and investigation of single solvent systems for delivery to the skin. Int. J. Pharm. X 2019, 1, 100025. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, F.; Hossain, A.S.M.M.A.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. Topical Delivery of 3-O-ethyl l-ascorbic Acid from Complex Solvent Systems. Sci. Pharm. 2020, 88, 19. [Google Scholar] [CrossRef] [Green Version]
- Kung, C.-P.; Zhang, Y.; Sil, B.C.; Hadgraft, J.; Lane, M.E.; Patel, B.; McCulloch, R. Investigation of binary and ternary solvent systems for dermal delivery of methadone. Int. J. Pharm. 2020, 586, 119538. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, I.; Müller-Goymann, C.C. Role of Isopropyl Myristate, Isopropyl Alcohol and a Combination of Both in Hydrocortisone Permeation across the Human Stratum corneum. Skin Pharmacol. Physiol. 2003, 16, 393–404. [Google Scholar] [CrossRef]
- Parisi, N.; Paz-Alvarez, M.; Matts, P.J.; Lever, R.; Hadgraft, J.; Lane, M.E. Topical delivery of hexamidine. Int. J. Pharm. 2016, 506, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Flach, C.R.; Mendelsohn, R.; Page, L.; Whitson, S.; Boncheva Bettex, M. Visualization of Epidermal Reservoir Formation from Topical Diclofenac Gels by Raman Spectroscopy. J. Pain Res. 2020, 13, 1621–1627. [Google Scholar] [CrossRef]
- Böhling, A.; Bielfeldt, S.; Himmelmann, A.; Keskin, M.; Wilhelm, K.P. Comparison of the stratum corneum thickness measured in vivo with confocal Raman spectroscopy and confocal reflectance microscopy. Skin Res. Technol. 2014, 20, 50–57. [Google Scholar] [CrossRef]
- Signorell, A.; Aho, K.; Alfons, A.; Anderegg, N.; Aragon, T.; Arachchige, C.; Arppe, A.; Baddeley, A.; Barton, K.; Bolker, B.; et al. DescTools: Tools for Descriptive Statistics. Available online: https://cran.r-project.org/package=DescTools (accessed on 8 June 2022).
- Krombholz, R.; Fressle, S.; Lunter, D. Ex vivo—In vivo correlation of retinol stratum corneum penetration studies by confocal Raman microspectroscopy and tape stripping. Int. J. Cosmet. Sci. 2022, 44, 299–308. [Google Scholar] [CrossRef]
- Barbero, A.M.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. Vitr. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- van de Sandt, J.J.M.; Meuling, W.J.A.; Elliott, G.R.; Cnubben, N.H.P.; Hakkert, B.C. Comparative in Vitro–in Vivo Percutaneous Absorption of the Pesticide Propoxur. Toxicol. Sci. 2000, 58, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Molen, R.G.; Spies, F.; van ‘t Noordende, J.M.; Boelsma, E.; Mommaas, A.M.; Koerten, H.K. Tape stripping of human stratum corneum yields cell layers that originate from various depths because of furrows in the skin. Arch. Dermatol. Res. 1997, 289, 514–518. [Google Scholar] [CrossRef] [PubMed]




| Diclac® Lipogel 10 mg/g | Primofenac® Emulsion gel 1% |
|---|---|
| DFNa | DFNa |
| RRR-α-tocopherol | cetyl alcohol |
| carbomer 980 NF | methyl-4-hydroxuybenzoate |
| decyl oleate | propyl-4-hydroxybenzoate |
| 2-octyldodecanol | isopropyl alcohol |
| Lecithin | glycerol |
| ammonium hydroxide 10% | polyacrylic acid (Carbomer) |
| disodium edetate | medium-chain triglycerides |
| perfume oil ’Vert de Creme’ | macrogol cetostearyl ether |
| isopropyl alcohol | purified water |
| purified water |
| Formulation | In vitro | In vivo | ||||
|---|---|---|---|---|---|---|
| Q24 (µg/cm2) | Jss (µg/cm2/h) | tlag (h) | AUCCRS (AU) | CRS intensity at 0.25 x/h (AU) | QTS (µg) | |
| Diclac | 86.5 ± 9.4 | 4.8 ± 0.5 | 6.0 ± 0.8 | 16.0 ± 2.9 | 38.2 ± 5.2 | 94.1 ± 22.6 |
| Primofenac | 24.4 ± 2.7 | 1.1 ± 0.1 | 1.7 ± 0.6 | 4.5 ± 1.0 | 10.9 ± 2.0 | 20.2 ± 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iliopoulos, F.; Goh, C.F.; Haque, T.; Rahma, A.; Lane, M.E. Dermal Delivery of Diclofenac Sodium—In Vitro and In Vivo Studies. Pharmaceutics 2022, 14, 2106. https://doi.org/10.3390/pharmaceutics14102106
Iliopoulos F, Goh CF, Haque T, Rahma A, Lane ME. Dermal Delivery of Diclofenac Sodium—In Vitro and In Vivo Studies. Pharmaceutics. 2022; 14(10):2106. https://doi.org/10.3390/pharmaceutics14102106
Chicago/Turabian StyleIliopoulos, Fotis, Choon Fu Goh, Tasnuva Haque, Annisa Rahma, and Majella E. Lane. 2022. "Dermal Delivery of Diclofenac Sodium—In Vitro and In Vivo Studies" Pharmaceutics 14, no. 10: 2106. https://doi.org/10.3390/pharmaceutics14102106
APA StyleIliopoulos, F., Goh, C. F., Haque, T., Rahma, A., & Lane, M. E. (2022). Dermal Delivery of Diclofenac Sodium—In Vitro and In Vivo Studies. Pharmaceutics, 14(10), 2106. https://doi.org/10.3390/pharmaceutics14102106