Development of Sedative Dexmedetomidine Sublingual In Situ Gels: In Vitro and In Vivo Evaluations
Abstract
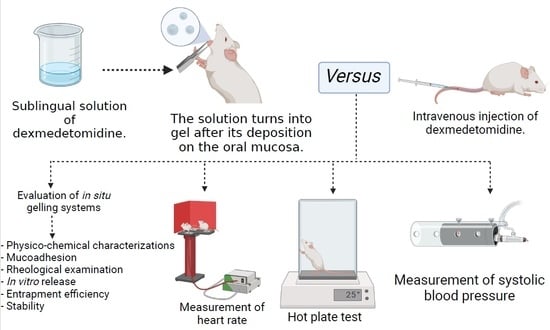
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of In Situ Gelling Systems
2.3. Evaluation of the Formulated In Situ GELLING Systems
2.3.1. Physicochemical Characterizations
2.3.2. Mucoadhesion
2.3.3. Rheological Examination
2.3.4. In Vitro Drug Release
2.4. Stability Study
2.5. In Vivo Pharmacokinetics Studies
2.6. Pharmacodynamics Studies
2.6.1. Hot Plate Test
2.6.2. Cardiovascular Effects
Measurement of Blood Pressure
Measurement of Heart Rate
2.7. Statistical Analysis
3. Results and Discussion
3.1. Formulation and Characterization of In Situ Gelling System for Sublingual Administration of DEX
3.2. In Vitro Drug Release
3.3. Stability Study
3.4. Pharmacokinetics Analysis
3.5. Pharmacodynamic Studies
3.5.1. Hot Plate Test
3.5.2. Measurement of Systolic Blood Pressure
3.5.3. Measurement of the Heart Rate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weerink, M.A.; Struys, M.M.; Hannivoort, L.N.; Barends, C.R.; Absalom, A.R.; Colin, P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [CrossRef] [Green Version]
- Lee, S. Dexmedetomidine: Present and future directions. Korean J. Anesthesiol. 2019, 72, 323–330. [Google Scholar] [CrossRef]
- Allam, A.; Elsabahy, M.; El Badry, M.; Eleraky, N.E. Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma. Int. J. Pharm. 2021, 598, 120380. [Google Scholar] [CrossRef]
- Allam, A.; Fetih, G. Sublingual fast dissolving niosomal films for enhanced bioavailability and prolonged effect of metoprolol tartrate. Drug Des. Devel. Ther. 2016, 10, 2421–2433. [Google Scholar]
- Youssef, A.; Dudhipala, N.; Majumdar, S. Ciprofloxacin Loaded Nanostructured Lipid Carriers Incorporated into In-Situ Gels to Improve Management of Bacterial Endophthalmitis. Pharmaceutics 2020, 12, 572. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariépy, E.; Leroux, J.C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- Srividya, B.; Cardoza, R.M.; Amin, P.D. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J. Control Release 2001, 73, 205–211. [Google Scholar] [CrossRef]
- Palhal, A.P. In-situ nasal gel: Modernistic advancement in drug delivery. World J. Pharm. Res. 2017, 6, 566–577. [Google Scholar] [CrossRef] [Green Version]
- Allam, A.; El-Mokhtar, M.A.; Elsabahy, M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J. Pharm. Pharmacol. 2019, 71, 1209–1221. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Streisand, J.B. Oral mucosal drug delivery: Clinical pharmacokinetics and therapeutic applications. Clin. Pharmacokinet. 2002, 41, 661–680. [Google Scholar] [CrossRef]
- Morales, J.O.; Fathe, K.R.; Brunaugh, A.; Ferrati, S.; Li, S.; Montenegro-Nicolini, M.; Mousavikhamene, Z.; McConville, J.T.; Prausnitz, M.R.; Smyth, H.D.C. Challenges and Future Prospects for the Delivery of Biologics: Oral Mucosal, Pulmonary, and Transdermal Routes. AAPS J. 2017, 19, 652–668. [Google Scholar] [CrossRef]
- Goyal, A.K.; Singh, R.; Chauhan, G.; Rath, G. Non-invasive systemic drug delivery through mucosal routes. Artif. Cells Nanomed. Biotechnol. 2018, 46, 539–551. [Google Scholar] [CrossRef] [Green Version]
- Hua, S. Advances in Nanoparticulate Drug Delivery Approaches for Sublingual and Buccal Administration. Front. Pharmacol. 2019, 10, 1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, S.A.B.; Abdel-Ghaffar, H.S.; Hassan, N.A.A.; El Sherif, F.A.; Shouman, S.A.; Omran, M.M.; Hassan, S.B.; Allam, A.A.A.E.M.; Sayed, D.G. Pharmacokinetics and Pharmacodynamics of 3 Doses of Oral-Mucosal Dexmedetomidine Gel for Sedative Premedication in Women Undergoing Modified Radical Mastectomy for Breast Cancer. Anesth. Analg. 2021, 132, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G.; Jain, S. Development and characterization of 99mTc-timolol maleate for evaluating efficacy of in situ ocular drug delivery system. AAPS PharmSciTech 2009, 10, 540–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kortesuo, P.; Ahola, M.; Kangas, M.; Jokinen, M.; Leino, T.; Vuorilehto, L.; Laakso, S.; Kiesvaara, J.; Yli-Urpo, A.; Marvola, M. Effect of synthesis parameters of the sol-gel-processed spray-dried silica gel microparticles on the release rate of dexmedetomidine. Biomaterials 2002, 23, 2795–2801. [Google Scholar] [CrossRef]
- Fathi, H.A.; Abdelkader, A.; AbdelKarim, M.S.; Abdelaziz, A.A.; El Mokhtar, M.A.; Allam, A.; Fetih, G.; El Badry, M.; Elsabahy, M. Electrospun vancomycin-loaded nanofibers for management of methicillin-resistant Staphylococcus aureus-induced skin infections. Int. J. Pharm. 2020, 586, 119620. [Google Scholar] [CrossRef]
- Saafan, H.A.; Ibrahim, K.M.; Thabet, Y.; Elbeltagy, S.M.; Eissa, R.A.; Ghaleb, A.H.; Ibrahim, F.; Elsabahy, M.; Eissa, N.G. Intratracheal administration of chloroquine-loaded niosomes minimize systemic drug exposure. Pharmaceutics 2021, 13, 1677. [Google Scholar] [CrossRef]
- El-awdan, S.A.W.; Al-shafeey, N.; Salam, O.A.; El-iraqy, W.I.; Kenawy, S.A.B.; Kenawy, B. Modulation of the pharmacological properties of meloxicam by octreotide in rats. J. Saudi Chem. Soc. 2015, 19, 123–132. [Google Scholar] [CrossRef] [Green Version]
- AboulFotouh, K.; Allam, A.A.; El-Badry, M.; El-Sayed, A.M. Development and in vitro/in vivo performance of self-nanoemulsifying drug delivery systems loaded with candesartan cilexetil. Eur. J. Pharm. Sci. 2017, 109, 503–513. [Google Scholar] [CrossRef]
- Abou El Ela, A.E.S.F.; Allam, A.A.; Ibrahim, E.H. Pharmacokinetics and anti-hypertensive effect of metoprolol tartrate rectal delivery system. Drug Deliv. 2016, 23, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Irimia, T.; Dinu-Pîrvu, C.-E.; Ghica, M.; Lupuleasa, D.; Muntean, D.-L.; Udeanu, D.; Popa, L. Chitosan-Based In Situ Gels for Ocular Delivery of Therapeutics: A State-of-the-Art Review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy HV, R.; Bhattacharyya, S. In vitro evaluation of mucoadhesive in situ nanogel of celecoxib for buccal delivery. Ann. Pharm. Françaises 2021, 79, 418–430. [Google Scholar] [CrossRef]
- Kurakula, M.; Naveen, N.R. In Situ Gel Loaded with Chitosan-Coated Simvastatin Nanoparticles: Promising Delivery for Effective Anti-Proliferative Activity against Tongue Carcinoma. Mar. Drugs 2020, 18, 201. [Google Scholar] [CrossRef] [Green Version]
- Bansal, M.; Mittal, N.; Yadav, S.K.; Khan, G.; Mishra, B.; Nath, G. Clinical evaluation of thermoresponsive and mucoadhesive Chitosan in situ gel containing Levofloxacin and Metronidazole in the treatment of periodontal pockets—A split-mouth, clinical study. J. Pierre Fauchard Acad. 2016, 30, 6–14. [Google Scholar] [CrossRef]
- Gupta, H.; Velpandian, T.; Jain, S. Ion- and pH-activated novel in-situ gel system for sustained ocular drug delivery. J. Drug Target. 2010, 18, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Makwana, S.B.; Patel, V.A.; Parmar, S.J. Development and characterization of in-situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma Sci. 2016, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Rai, V.K.; Yadav, N.P.; Sinha, P.; Mishra, N.; Luqman, S.; Dwivedi, H.; Kymonil, K.M.; Saraf, S.A. Development of cellulosic polymer based gel of novel ternary mixture of miconazole nitrate for buccal delivery. Carbohydr. Polym. 2014, 103, 126–133. [Google Scholar] [CrossRef]
- Patil, S.; Kadam, A.; Bandgar, S.; Patil, S. Formulation and evaluation of an in situ gel for ocular drug delivery of anticonjunctival drug. Cellul. Chem. Technol. 2015, 49, 35–40. [Google Scholar]
- El-Badry, M.; Fetih, G.; Fathalla, D.; Shakeel, F. Transdermal delivery of meloxicam using niosomal hydrogels: In vitro and pharmacodynamic evaluation. Pharm. Dev. Technol. 2015, 20, 820–826. [Google Scholar] [CrossRef]
- Phaechamud, T.; Senarat, S.; Puyathorn, N.; Praphanwittaya, P. Solvent exchange and drug release characteristics of doxycycline hyclate-loaded bleached shellac in situ-forming gel and -microparticle. Int. J. Biol. Macromol. 2019, 135, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Bol, C.J.J.G.; Danhof, M.; Stanski, D.R.; Mandema, J.W. Pharmacokinetic-Pharmacodynamic Characterization of the Cardiovascular, Hypnotic, EEG and Ventilatory Responses to Dexmedetomidine in the Rat. J. Pharmacol. Exp. Ther. 1997, 283, 1051–1058. [Google Scholar] [PubMed]
- Anttila, M.; Penttilä, J.; Helminen, A.; Vuorilehto, L.; Scheinin, H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br. J. Clin. Pharmacol. 2003, 56, 691–693. [Google Scholar] [CrossRef] [Green Version]
- Penttilä, J.; Helminen, A.; Anttila, M.; Hinkka, S.; Scheinin, H. Cardiovascular and parasympathetic effects of dexmedetomidine in healthy subjects. Can. J. Physiol. Pharmacol. 2004, 82, 359–362. [Google Scholar] [CrossRef] [PubMed]






| Code | pH | Viscosity (Pa S) a (pH 6.8 ± 0.2, 37 °C) | Mucoadhesive Force (Pa) | Gelling Capacity b |
|---|---|---|---|---|
| F2 | 4.9 ± 0.2 | 26.54 ± 2.84 | 3.90 ± 1.68 | ++ |
| F3 | 5.1 ± 0.1 | 37.46 ± 0.82 | 4.83 ± 0.34 | +++ |
| F6 | 5.8 ± 0.1 | 23.74 ± 1.12 | 3.52 ± 2.51 | + |
| F7 | 6.0 ± 0.1 | 32.66 ± 2.13 | 3.81 ± 1.41 | ++ |
| F10 | 6.2 ± 0.3 | 17.50 ± 3.02 | 1.40 ± 1.33 | + |
| F11 | 6.4 ± 0.2 | 19.53 ± 2.97 | 1.82 ± 1.74 | + |
| F12 | 6.5 ± 0.2 | 24.56 ± 1.92 | 2.22 ± 2.24 | ++ |
| Formulation | Determination Coefficient (r2) | (n) Korsmeyer–Peppas Equation | |||
|---|---|---|---|---|---|
| Zero-Order | First-Order | Higuchi Diffusion | Peppas | ||
| F2 | 0.8409 | 0.2895 | 0.9580 | 0.9796 | 0.30 |
| F3 | 0.8729 | 0.15093 | 0.9739 | 0.9872 | 0.35 |
| F6 | 0.7863 | 0.34022 | 0.9267 | 0.9621 | 0.24 |
| F7 | 0.8455 | 0.2381 | 0.9595 | 0.9686 | 0.34 |
| F10 | 0.7552 | 0.6593 | 0.9064 | 0.9308 | 0.22 |
| F11 | 0.8032 | 0.5296 | 0.9369 | 0.971 | 0.25 |
| F12 | 0.7757 | 0.2864 | 0.9193 | 0.9279 | 0.26 |
| Time (d) | Zero Time | 30 d | 60 d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Storage Temperature | 4 °C | 25 °C | 40 °C | 4 °C | 25 °C | 40 °C | 4 °C | 25 °C | 40 °C |
| Surface pH * | 4.9 ± 0.22 | 5.1 ± 0.19 | 5.0 ± 0.23 | 5.2 ± 0.20 | 4.9 ± 0.19 | 5.0 ± 0.22 | 5.1 ± 0.21 | 5.0 ± 0.15 | 5.1 ± 0.18 |
| Viscosity (Pa S) (pH 6.8, 37 °C) | 38.16 ± 0.91 | 37.46 ± 0.82 | 35.99 ± 0.88 | 38.44 ± 0.99 | 37.16 ± 0.78 | 35.76 ± 0.69 | 38.56 ± 0.72 | 36.21 ± 0.92 | 35.96 ± 0.96 |
| Mucoadhesive force (Pa) | 4.91 ± 0.29 | 4.83 ± 0.34 | 4.72 ± 0.44 | 4.89 ± 0.31 | 4.79 ± 0.54 | 4.76 ± 0.33 | 4.93 ± 0.24 | 4.81 ± 0.25 | 4.7 ± 0.62 |
| Gelling Capacity | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Formulation | Pharmacokinetic Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Cmax (μg mL−1) | Tmax (min) | V/F (min−1) | Cl/F (min) | Kel (min−1) | t½ (min) | AUC (μg min mL−1) | F (%) | |
| DEX oral | 0.39 ± 0.05 | 120 ± 9.5 | 1.02 ± 0.21 | 0.013 ± 0.00 | 0.0013 ± 0.00 | 53.61 ± 2.24 | 74.19 ± 14.43 | 43.83 |
| DEX IV bolus | 0.86 ± 0.06 | - | 0.89 ± 0.06 | 0.005 ± 0.00 | 0.007 ± 0.00 | 99.81 ± 15.2 | 169.26 ± 20.025 * | 100 * |
| DEX sublingual (in situ gel F3) | 0.75 ± 0.05 | 60 ± 11.3 | 0.76 ± 0.05 | 0.006 ± 0.00 | 0.008 ± 0.00 | 87.12 ± 17.06 | 151.02 ± 17.27 * | 89.22 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allam, A.A.; Eleraky, N.E.; Diab, N.H.; Elsabahy, M.; Mohamed, S.A.; Abdel-Ghaffar, H.S.; Hassan, N.A.; Shouman, S.A.; Omran, M.M.; Hassan, S.B.; et al. Development of Sedative Dexmedetomidine Sublingual In Situ Gels: In Vitro and In Vivo Evaluations. Pharmaceutics 2022, 14, 220. https://doi.org/10.3390/pharmaceutics14020220
Allam AA, Eleraky NE, Diab NH, Elsabahy M, Mohamed SA, Abdel-Ghaffar HS, Hassan NA, Shouman SA, Omran MM, Hassan SB, et al. Development of Sedative Dexmedetomidine Sublingual In Situ Gels: In Vitro and In Vivo Evaluations. Pharmaceutics. 2022; 14(2):220. https://doi.org/10.3390/pharmaceutics14020220
Chicago/Turabian StyleAllam, Ayat A., Nermin E. Eleraky, Nadeen H. Diab, Mahmoud Elsabahy, Sahar A. Mohamed, Hala S. Abdel-Ghaffar, Nivin A. Hassan, Samia A. Shouman, Mervat M. Omran, Sahar B. Hassan, and et al. 2022. "Development of Sedative Dexmedetomidine Sublingual In Situ Gels: In Vitro and In Vivo Evaluations" Pharmaceutics 14, no. 2: 220. https://doi.org/10.3390/pharmaceutics14020220
APA StyleAllam, A. A., Eleraky, N. E., Diab, N. H., Elsabahy, M., Mohamed, S. A., Abdel-Ghaffar, H. S., Hassan, N. A., Shouman, S. A., Omran, M. M., Hassan, S. B., & Eissa, N. G. (2022). Development of Sedative Dexmedetomidine Sublingual In Situ Gels: In Vitro and In Vivo Evaluations. Pharmaceutics, 14(2), 220. https://doi.org/10.3390/pharmaceutics14020220