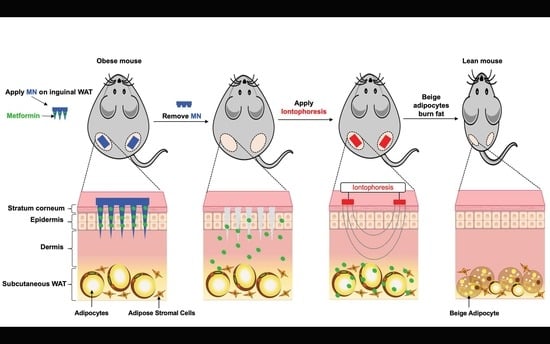
Transdermal Delivery of Metformin Using Dissolving Microneedles and Iontophoresis Patches for Browning Subcutaneous Adipose Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. MN Patch Fabrication
2.2. MN Patch Application Procedures
2.3. INT Application Procedures
2.4. In Vivo Fluorescence Imaging and Biodistribution of DID Dye Using MN Patches and INT
3. Anti-Obesity Mice Study
3.1. Animals
3.2. Body Composition
3.3. Indirect Calorimetry
3.4. Plasma Lipid Profile
3.5. GTT
3.6. IgWAT and Liver Metformin Content
3.7. Real-Time PCR
3.8. Immunohistochemistry
3.9. Hematoxylin and Eosin Staining
3.10. Statistical Analysis
4. Results
4.1. MN Derma-Rollers and INT-Based In Vitro Transdermal Delivery
4.2. The PLGA MN Patch
4.3. In Vivo Fluorescence Imaging and Biodistribution of DID Dye Using MN Patches and INT
4.3.1. Anti-Obesity and Metabolic Benefits of Metformin in HFD-Induced Obese C57BL/6J Mice
4.3.2. Browning Activities
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Acetyl-CoA carboxylase 1 | (ACC1) |
| AMP-activated protein kinase | (AMPK) |
| Cell death activator | (CIDEA) |
| Control | (no MN, no INT) |
| DID-loaded MN | (MN (DID) alone) |
| DID-loaded INT | (INT (DID) alone) |
| DID-loaded MN followed by INT | (MN (DID) + INT) |
| Dimethylformamide | (DMF) |
| Elongase of very long chain fatty acids-3 | (ELOVL3) |
| Glucose tolerance test | (GTT) |
| GTT area under the curve | (GTT-AUC) |
| Glucose transporter type 2 | (GLUT2) |
| Glycolic acid | (GA) |
| Gonadal WAT | (GWAT) |
| Hematoxylin and eosin | (H&E) |
| High-performance liquid chromatography | (HPLC) |
| high density lipoprotein | (HDL) |
| High fat diet | (HFD) |
| Inguinal WAT | (IgWAT) |
| Iontophoresis | (INT) |
| Lactic acid | (LA) |
| Low density lipoprotein | (LDL) |
| Metformin-loaded MN | (MN (met) alone) |
| Metformin-loaded INT | (INT (met) alone) |
| Metformin-loaded MN followed by INT | (MN (met) + INT) |
| Microneedles | (MN) |
| Monocyte chemoattractant protein 1 | (MCP1) |
| No metformin loaded in MN or INT | (MN + INT (blank)) |
| Phosphorylated AMPK | (pAMPK) |
| Poly (lactic-co-glycolic acid) | (PLGA) |
| Polydimethylsiloxane | (PDMS) |
| PR domain containing 16 | (PRDM16) |
| Phosphoenolpyruvate carboxykinase | (PEPCK) |
| Peroxisome proliferator-activated receptor gamma coactivator 1-alpha | (PGC1) |
| Respiratory exchange ratio | (RER) |
| Scanning electron microscope | (SEM) |
| Sirtuin (silent mating type information regulation 2 homolog) 1 | (SIRT1) |
| Transmembrane protein 26 | (TMEM26) |
| Tumor necrosis factor | (TNFα) |
| Uncoupling protein 1 | (UCP1) |
| Very low-density lipoprotein | (VLDL) |
| White adipose tissue | (WAT) |
| Zic Family Member 1 | (ZIC1) |
| 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt | (DID) |
| 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (ammonium salt) | (Rohd-PE) |
References
- Adami, G.F.; Carbone, F.; Montecucco, F.; Camerini, G.; Cordera, R. Adipose Tissue Composition in Obesity and after Bariatric Surgery. Obes. Surg. 2019, 29, 3030–3038. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Swarbrick, M.M.; Ho, K.K. Brown adipose tissue in adult humans: A metabolic renaissance. Endocr. Rev. 2013, 34, 413–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Lidell, M.E.; Enerbäck, S. Brown adipose tissue—A new role in humans? Nat. Rev. Endocrinol. 2010, 6, 319–325. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Cypess, A.M.; Kahn, C.R. Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 2010, 9, 465–482. [Google Scholar] [CrossRef] [Green Version]
- Kaisanlahti, A.; Glumoff, T. Browning of white fat: Agents and implications for beige adipose tissue to type 2 diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Nedergaard, J.; Cannon, B. The Browning of White Adipose Tissue: Some Burning Issues. Cell Metab. 2014, 20, 396–407. [Google Scholar] [CrossRef] [Green Version]
- Wankhade, U.D.; Shen, M.; Yadav, H.; Thakali, K.M. Novel Browning Agents, Mechanisms, and Therapeutic Potentials of Brown Adipose Tissue. Biomed. Res. Int. 2016, 2016, 2365609. [Google Scholar] [CrossRef] [Green Version]
- Abdullahi, A.; Jeschke, M.G. Taming the Flames: Targeting White Adipose Tissue Browning in Hypermetabolic Conditions. Endocr. Rev. 2017, 38, 538–549. [Google Scholar] [CrossRef]
- Malin, S.K.; Kashyap, S.R. Effects of metformin on weight loss: Potential mechanisms. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Goswami, S.; Giacomini, K.M.; Altman, R.B.; Klein, T.E. Metformin pathways: Pharmacokinetics and pharmacodynamics. Pharm. Genom. 2012, 22, 820–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saluja, M.; Pareek, K.K.; Swami, Y.K. Study of Diversity of Metformin Related Gastrointestinal Side Effects. J. Assoc. Physicians India 2020, 68, 36–38. [Google Scholar] [PubMed]
- Than, A.; Liang, K.; Xu, S.; Sun, L.; Duan, H.; Xi, F.; Xu, C.; Chen, P. Transdermal Delivery of Anti-Obesity Compounds to Subcutaneous Adipose Tissue with Polymeric Microneedle Patches. Small Methods 2017, 1, 1700269. [Google Scholar] [CrossRef]
- Garland, M.J.; Caffarel-Salvador, E.; Migalska, K.; Woolfson, A.D.; Donnelly, R.F. Dissolving polymeric microneedle arrays for electrically assisted transdermal drug delivery. J. Control. Release 2012, 159, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.; Prausnitz, M.R.; Mitragotri, S. Micro-scale devices for transdermal drug delivery. Int. J. Pharm. 2008, 364, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Chu, L.Y.; Choi, S.O.; Prausnitz, M.R. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: Bubble and pedestal microneedle designs. J. Pharm. Sci. 2010, 99, 4228–4238. [Google Scholar] [CrossRef]
- Hong, X.; Wei, L.; Wu, F.; Wu, Z.; Chen, L.; Liu, Z.; Yuan, W. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des. Dev. Ther. 2013, 7, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Yao, G.; Dong, P.; Gong, Z.; Li, G.; Zhang, K.; Wu, C. Investigation on fabrication process of dissolving microneedle arrays to improve effective needle drug distribution. Eur. J. Pharm. Sci. 2015, 66, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Naves, L.; Dhand, C.; Almeida, L.; Rajamani, L.; Ramakrishna, S.; Soares, G. Poly(lactic-co-glycolic) acid drug delivery systems through transdermal pathway: An overview. Prog. Biomater. 2017, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Dhote, V.; Bhatnagar, P.; Mishra, P.K.; Mahajan, S.C.; Mishra, D.K. Iontophoresis: A potential emergence of a transdermal drug delivery system. Sci. Pharm. 2012, 80, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D. Quantifying Chromogen Intensity in Immunohistochemistry via Reciprocal Intensity. 2013. Available online: https://protocolexchange.researchsquare.com/article/nprot-2931/v1.
- Kaur, G.; Grewal, J.; Jyoti, K.; Jain, U.K.; Chandra, R.; Madan, J. Chapter 15—Oral controlled and sustained drug delivery systems: Concepts, advances, preclinical, and clinical status. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 567–626. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.; Lee, S.J. Effective method for drug injection into subcutaneous tissue. Sci. Rep. 2017, 7, 9613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Yu, J.; Yu, S.; Wang, J.; Qiang, L.; Gu, Z. Locally Induced Adipose Tissue Browning by Microneedle Patch for Obesity Treatment. ACS Nano 2017, 11, 9223–9230. [Google Scholar] [CrossRef]
- Dangol, M.; Kim, S.; Li, C.G.; Fakhraei Lahiji, S.; Jang, M.; Ma, Y.; Huh, I.; Jung, H. Anti-obesity effect of a novel caffeine-loaded dissolving microneedle patch in high-fat diet-induced obese C57BL/6J mice. J. Control. Release 2017, 265, 41–47. [Google Scholar] [CrossRef]
- Karpiński, T.M. Selected Medicines Used in Iontophoresis. Pharmaceutics 2018, 10, 204. [Google Scholar] [CrossRef] [Green Version]
- Katikaneni, S.; Badkar, A.; Nema, S.; Banga, A.K. Molecular charge mediated transport of a 13 kD protein across microporated skin. Int. J. Pharm. 2009, 378, 93–100. [Google Scholar] [CrossRef]
- Arayne, M.S.; Sultana, N.; Zuberi, M.H. Development and validation of RP-HPLC method for the analysis of metformin. Pak. J. Pharm. Sci. 2006, 19, 231–235. [Google Scholar]
- Yuan, T.; Li, J.; Zhao, W.G.; Sun, W.; Liu, S.N.; Liu, Q.; Fu, Y.; Shen, Z.F. Effects of metformin on metabolism of white and brown adipose tissue in obese C57BL/6J mice. Diabetol. Metab. Syndr. 2019, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Karise, I.; Bargut, T.C.; del Sol, M.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Metformin enhances mitochondrial biogenesis and thermogenesis in brown adipocytes of mice. Biomed. Pharmacother. 2019, 111, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Kinaan, M.; Ding, H.; Triggle, C.R. Metformin: An Old Drug for the Treatment of Diabetes but a New Drug for the Protection of the Endothelium. Med. Princ. Pract. 2015, 24, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Igel, L.I.; Sinha, A.; Saunders, K.H.; Apovian, C.M.; Vojta, D.; Aronne, L.J. Metformin: An Old Therapy that Deserves a New Indication for the Treatment of Obesity. Curr. Atheroscler. Rep. 2016, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Desilets, A.R.; Dhakal-Karki, S.; Dunican, K.C. Role of Metformin for Weight Management in Patients Without Type 2 Diabetes. Ann. Pharmacother. 2008, 42, 817–826. [Google Scholar] [CrossRef]
- Migdadi, E.M.; Courtenay, A.J.; Tekko, I.A.; McCrudden, M.T.C.; Kearney, M.-C.; McAlister, E.; McCarthy, H.O.; Donnelly, R.F. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J. Control. Release 2018, 285, 142–151. [Google Scholar] [CrossRef]
- Gonzalez-Hurtado, E.; Lee, J.; Choi, J.; Wolfgang, M.J. Fatty acid oxidation is required for active and quiescent brown adipose tissue maintenance and thermogenic programing. Mol. Metab. 2018, 7, 45–56. [Google Scholar] [CrossRef]
- Gaidhu, M.P.; Anthony, N.M.; Patel, P.; Hawke, T.J.; Ceddia, R.B. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: Role of ATGL, HSL, and AMPK. Am. J. Physiol.-Cell Physiol. 2010, 298, C961–C971. [Google Scholar] [CrossRef] [Green Version]
- López-Ibarra, Z.; Modrego, J.; Valero-Muñoz, M.; Rodríguez-Sierra, P.; Zamorano-León, J.J.; González-Cantalapiedra, A.; Heras, N.d.l.; Ballesteros, S.; Lahera, V.; López-Farré, A.J. Metabolic differences between white and brown fat from fasting rabbits at physiological temperature. J. Mol. Endocrinol. 2015, 54, 105. [Google Scholar] [CrossRef] [Green Version]
- Braun, K.; Oeckl, J.; Westermeier, J.; Li, Y.; Klingenspor, M. Non-adrenergic control of lipolysis and thermogenesis in adipose tissues. J. Exp. Biol. 2018, 221 (Suppl. 1), jeb165381. [Google Scholar] [CrossRef] [Green Version]
- Scheja, L.; Heeren, J. Metabolic interplay between white, beige, brown adipocytes and the liver. J. Hepatol. 2016, 64, 1176–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeke, G.; Kooijman, S.; Boon, M.R.; Rensen, P.C.N.; Berbée, J.F.P. Role of Brown Fat in Lipoprotein Metabolism and Atherosclerosis. Circ. Res. 2016, 118, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Lee, S.H.; Jhun, J.Y.; Byun, J.K.; Jeong, J.H.; Lee, S.-Y.; Kim, J.K.; Choi, J.Y.; Cho, M.-L. Metformin Prevents Fatty Liver and Improves Balance of White/Brown Adipose in an Obesity Mouse Model by Inducing FGF21. Mediat. Inflamm. 2016, 2016, 5813030. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef] [Green Version]
- Cantó, C.; Auwerx, J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol. Life Sci. 2010, 67, 3407–3423. [Google Scholar] [CrossRef] [Green Version]
- Auger, C.; Knuth, C.M.; Abdullahi, A.; Samadi, O.; Parousis, A.; Jeschke, M.G. Metformin prevents the pathological browning of subcutaneous white adipose tissue. Mol. Metab. 2019, 29, 12–23. [Google Scholar] [CrossRef]
- Kola, B.; Grossman, A.B.; Korbonits, M. The role of AMP-activated protein kinase in obesity. In Obesity and Metabolism; Karger Publisher: Basel, Switzerland, 2008; Volume 36, pp. 198–211. [Google Scholar]
- Long, Y.C.; Zierath, J.R. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Investig. 2006, 116, 1776–1783. [Google Scholar] [CrossRef] [Green Version]
- Breining, P.; Jensen, J.B.; Sundelin, E.I.; Gormsen, L.C.; Jakobsen, S.; Busk, M.; Rolighed, L.; Bross, P.; Fernandez-Guerra, P.; Markussen, L.K.; et al. Metformin targets brown adipose tissue in vivo and reduces oxygen consumption in vitro. Diabetes Obes. Metab. 2018, 20, 2264–2273. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, X.; Sun, X.; Zhang, L.; Fu, X.; Rogers, C.J.; Berim, A.; Zhang, S.; Wang, S.; Wang, B.; et al. AMPK/α-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell Metab. 2016, 24, 542–554. [Google Scholar] [CrossRef] [Green Version]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Corrêa, L.H.; Heyn, G.S.; Magalhaes, K.G. The Impact of the Adipose Organ Plasticity on Inflammation and Cancer Progression. Cells 2019, 8, 662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smorlesi, A.; Frontini, A.; Giordano, A.; Cinti, S. The adipose organ: White-brown adipocyte plasticity and metabolic inflammation. Obes. Rev. 2012, 13 (Suppl. 2), 83–96. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Inflammation versus Host Defense in Obesity. Cell Metab. 2014, 20, 708–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porta, V.; Schramm, S.G.; Kano, E.K.; Koono, E.E.; Armando, Y.P.; Fukuda, K.; Serra, C.H.D.R. HPLC-UV determination of metformin in human plasma for application in pharmacokinetics and bioequivalence studies. J. Pharm. Biomed. Anal. 2008, 46, 143–147. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi, M.; Fan, Z.; Dawson, J.A.; Wang, S. Transdermal Delivery of Metformin Using Dissolving Microneedles and Iontophoresis Patches for Browning Subcutaneous Adipose Tissue. Pharmaceutics 2022, 14, 879. https://doi.org/10.3390/pharmaceutics14040879
Abbasi M, Fan Z, Dawson JA, Wang S. Transdermal Delivery of Metformin Using Dissolving Microneedles and Iontophoresis Patches for Browning Subcutaneous Adipose Tissue. Pharmaceutics. 2022; 14(4):879. https://doi.org/10.3390/pharmaceutics14040879
Chicago/Turabian StyleAbbasi, Mehrnaz, Zhaoyang Fan, John A. Dawson, and Shu Wang. 2022. "Transdermal Delivery of Metformin Using Dissolving Microneedles and Iontophoresis Patches for Browning Subcutaneous Adipose Tissue" Pharmaceutics 14, no. 4: 879. https://doi.org/10.3390/pharmaceutics14040879
APA StyleAbbasi, M., Fan, Z., Dawson, J. A., & Wang, S. (2022). Transdermal Delivery of Metformin Using Dissolving Microneedles and Iontophoresis Patches for Browning Subcutaneous Adipose Tissue. Pharmaceutics, 14(4), 879. https://doi.org/10.3390/pharmaceutics14040879