Combination of Lanosterol and Nilvadipine Nanosuspensions Rescues Lens Opacification in Selenite-Induced Cataractic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Preparation of LAN-ONSs and NIL-ONSs
2.4. Measurement of LAN and NIL
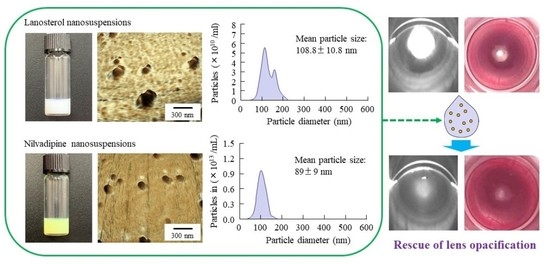
2.5. Evaluation of Drug Particles in LAN-ONSs and NIL-ONSs
2.6. Measurement of Drug Content in the Lenses
2.7. Assessment of Lens Opacification via Imaging
2.8. Evaluation of Cataract-Related Factors
2.9. Evaluation of Lens Structure in Selenite-Induced Cataractic Rats Using Hematoxylin and Eosin (H.E.) Staining
2.10. Statistical Analysis
3. Results
3.1. Drug Delivery to the Lens by Instillation of LAN-ONSs and NIL-ONSs
3.2. Improvement of Lens Opacification in Sodium-Selenite-Induced Cataractic Rats by Repeated Co-Instillation of LAN-ONSs and NIL-ONSs
3.3. Changes in the Cataract-Related Factors of Sodium-Selenite-Induced Cataractic Rat by the Co-Instillation of LAN-ONSs and NIL-ONSs
3.4. Changes in the Lens Structure of Sodium-Selenite-Induced Cataractic Rat by the Co-Instillation of LAN-ONSs and NIL-ONSs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nirmalan, P.K.; Krishnadas, R.; Ramakrishnan, R.; Thulasiraj, R.D.; Katz, J.; Tielsch, J.M.; Robin, A.L. Lens opacities in a rural population of southern India: The Aravind Comprehensive Eye Study. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4639–4643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murthy, G.V.S.; Gupta, S.K.; Maraini, G.; Camparini, M.; Price, G.M.; Dherani, M.; John, N.; Chakravarthy, U.; Fletcher, A.E. Prevalence of lens opacities in North India: The INDEYE feasibility study. Investig. Ophthalmol. Vis. Sci. 2007, 48, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Michael, R.; Bron, A.J. The ageing lens and cataract: A model of normal and pathological ageing. Phil. Trans. R. Soc. B 2011, 366, 1278–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, K.L.; King, J.A. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol. Med. 2012, 18, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brian, G.; Taylor, H. Cataract blindness–challenges for the 21st century. Bull. World Health Organ. 2001, 79, 249–256. [Google Scholar]
- Kruk, J.; Kubasik-Kladna, K.; Aboul-Enein, H.Y. The Role Oxidative Stress in the Pathogenesis of Eye Diseases: Current Status and a Dual Role of Physical Activity. Mini Rev. Med. Chem. 2015, 16, 241–257. [Google Scholar] [CrossRef]
- World Health Organization. Vision 2020 the Right to Sight. Global Initiative for the Elimination of Avoidable Blindness. Available online: https://www.who.int/blindness/Vision2020_report.pdf (accessed on 7 June 2022).
- Chang, M.A.; Congdon, N.G.; Baker, S.K.; Bloem, M.W.; Savage, H.; Sommer, A. The surgical management of cataract: Barriers, best practices and outcomes. Int. Ophthalmol. 2008, 28, 247–260. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, X.-J.; Zhu, J.; Xi, Y.-B.; Yang, X.; Hu, L.-D.; Ouyang, H.; Patel, S.H.; Jin, X.; Lin, D.; et al. Lanosterol reverses protein aggregation in cataracts. Nature 2015, 523, 607–611. [Google Scholar] [CrossRef]
- Makley, L.N.; McMenimen, K.A.; DeVree, B.T.; Goldman, J.W.; McGlasson, B.N.; Rajagopal, P.; Dunyak, B.M.; McQuade, T.J.; Thompson, A.D.; Sunahara, R.; et al. Pharmacological chaperone for alpha-crystallin partially restores transparency in cataract models. Science 2015, 350, 674–677. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Umachi, K.; Otake, H.; Oka, M.; Hiramatsu, N.; Sasaki, H.; Yamamoto, N. Ophthalmic In Situ Gelling System containing Lanosterol Nanoparticles Delays Collapse of Lens Structure in Shumiya Cataract Rats. Pharmaceutics 2020, 12, 629. [Google Scholar] [CrossRef]
- Harding, J.J. Cataract: Biochemistry, Epidemiology and Pharmacology, 1st ed.; Chapman and Hall, Ltd.: London, UK, 1991; pp. 125–194. [Google Scholar]
- Sanderson, J.; Marcantonio, J.M.; Duncan, G. A human lens model of cortical cataract: Ca2+-induced protein loss, vimentin cleavage and opacification. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2255–2261. [Google Scholar]
- Shearer, T.R.; Ma, H.; Fukiage, C.; Azuma, M. Selenite nuclear cataract: Review of the model. Mol. Vis. 1997, 23, 8–17. [Google Scholar]
- Nakamura, Y.; Fukiage, C.; Shih, M.; Ma, H.; David, L.L.; Azuma, M.; Shearer, T.R. Contribution of calpain Lp82-induced proteolysis to experimental cataractogenesis in mice. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1460–1466. [Google Scholar]
- Truscott, R.J.; Marcantonio, J.M.; Tomlinson, J.; Duncan, G. Calcium-induced opacification and proteolysis in the intact rat lens. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2405–2411. [Google Scholar]
- Goto, R.; Yamada, S.; Otake, H.; Nakazawa, Y.; Oka, M.; Yamamoto, N.; Sasaki, H.; Nagai, N. Instillation of Ophthalmic Formulation Containing Nilvadipine Nanocrystals Attenuates Lens Opacification in Shumiya Cataract Rats. Pharmaceutics 2021, 13, 1999. [Google Scholar] [CrossRef]
- Xiaoguang, W.; Jianjun, C.; Qinying, C.; Hui, Z.; Lukun, Y.; Yazhen, S. Establishment of a Valuable Mimic of Alzheimer’s Disease in Rat Animal Model by Intracerebroventricular Injection of Composited Amyloid Beta Protein. J. Vis. Exp. 2018, 137, 56157. [Google Scholar] [CrossRef]
- Swearengen, J.R. Choosing the right animal model for infectious disease research. Anim. Model. Exp. Med. 2018, 1, 100–108. [Google Scholar] [CrossRef]
- Tolbert, W.D.; Subedi, G.P.; Gohain, N.; Lewis, G.K.; Patel, K.R.; Barb, A.W.; Pazgier, M. From Rhesus macaque to human: Structural evolutionary pathways for immunoglobulin G subclasses. mAbs 2019, 11, 709–724. [Google Scholar] [CrossRef]
- Shearer, T.R.; David, L.L.; Anderson, R.A. Selenite cataract: A review. Curr. Eye Res. 1987, 6, 289–300. [Google Scholar] [CrossRef]
- Bunce, G.E.; Hess, J.L. Biochemical changes associated with selenite-induced cataract in the rat. Exp. Eye Res. 1981, 35, 505–514. [Google Scholar] [CrossRef]
- Ostádalová, I.; Babický, A.; Obenberger, J. Cataract induced by administration of a single dose of sodium selenite to suckling rats. Experientia 1978, 34, 222–223. [Google Scholar] [CrossRef] [PubMed]
- David, L.L.; Shearer, T.R. Beta-crystallins insolubilized by calpain II in vitro contain cleavage sites similar to beta-crystallins insolubilized during cataract. FEBS Lett. 1993, 32, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Fukuoka, Y.; Sato, K.; Otake, H.; Taga, A.; Oka, M.; Hiramatsu, N.; Yamamoto, N. The Intravitreal Injection of Lanosterol Nanoparticles Rescues Lens Structure Collapse at an Early Stage in Shumiya Cataract Rats. Int. J. Mol. Sci. 2020, 21, 1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, N.; Ito, Y.; Takeuchi, N. Inhibitive effects of enhanced lipid peroxidation on Ca2+-ATPase in lenses of hereditary cataract ICR/f rats. Toxicology 2008, 247, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Takeuchi, N.; Usui, S.; Hirano, K. Comparison of the Mechanisms of Cataract Development Involving Differences in Ca2+ Regulation in Lenses among Three Hereditary Cataract Model Rats. Biol. Pharm. Bull. 2008, 31, 1990–1995. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef]
- Hagigit, T.; Abdulrazik, M.; Orucov, F.; Valamanesh, F.; Hagedorn, M.; Lambert, G.; Behar-Cohen, F.; Benita, S. Topical and intravitreous administration of cationic nanoemulsions to deliver antisense oligonucleotides directed towards VEGF KDR receptors to the eye. J. Control. Release 2010, 145, 297–305. [Google Scholar] [CrossRef]
- Hironaka, K.; Inokuchi, Y.; Fujisawa, T.; Shimazaki, H.; Akane, M.; Tozuka, Y.; Tsuruma, K.; Shimazawa, M.; Hara, H.; Takeuchi, H. Edaravone-loaded liposomes for retinal protection against oxidative stress-induced retinal damage. Eur. J. Pharm. Biopharm. 2011, 79, 119–125. [Google Scholar] [CrossRef]
- Gan, L.; Gan, Y.; Zhu, C.; Zhang, X.; Zhu, J. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: In vitro and in vivo results. Int. J. Pharm. 2009, 365, 143–149. [Google Scholar] [CrossRef]
- Sun, D.; Maeno, H.; Gujrati, M.; Schur, R.; Maeda, A.; Maeda, T.; Palczewski, K.; Lu, Z.-R. Self-Assembly of a Multifunctional Lipid with Core-Shell Dendrimer DNA Nanoparticles Enhanced Efficient Gene Delivery at Low Charge Ratios into RPE Cells. Macromol. Biosci. 2015, 15, 1663–1672. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Y.; Ping, Q.; Xiao, Y.; Huang, X. Mucoadhesive effect of thiolated PEG stearate and its modified NLC for ocular drug delivery. J. Control. Release 2009, 137, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Tanino, T.; Ito, Y.; Okamoto, N.; Shimomura, Y. Decrease in Corneal Damage due to Benzalkonium Chloride by the Addition of Mannitol into Timolol Maleate Eye Drops. J. Oleo Sci. 2015, 64, 743–750. [Google Scholar] [CrossRef] [Green Version]
- David, L.L.; Shearer, T.R. State of sulfhydryl in selenite cataract. Toxicol. Appl. Pharmacol. 1984, 74, 109–115. [Google Scholar] [CrossRef]
- Kuroki, T.; Mori, A.; Nakahara, T.; Sakamoto, K.; Ishii, K. Histological protection by nilvadipine against neurotoxicity induced by NOC12, a nitric oxide donor, in the rat retina. Biol. Pharm. Bull. 2014, 37, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Hightower, K.R.; David, L.L.; Shearer, T.R. Regional distribution of free calcium in selenite cataract: Relation to calpain II. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1702–1706. [Google Scholar]








| Formulation | Composition (%w/v) | ||||||
|---|---|---|---|---|---|---|---|
| LAN | NIL | HPβCD | MC | BAC | Mannitol | Treatment | |
| LAN-ONSs | 0.5 | – | 5 | 0.5 | 0.001 | 0.5 | Bead mill |
| NIL-ONSs | – | 0.6 | 5 | 0.5 | 0.001 | 0.5 | Bead mill |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deguchi, S.; Kadowaki, R.; Otake, H.; Taga, A.; Nakazawa, Y.; Misra, M.; Yamamoto, N.; Sasaki, H.; Nagai, N. Combination of Lanosterol and Nilvadipine Nanosuspensions Rescues Lens Opacification in Selenite-Induced Cataractic Rats. Pharmaceutics 2022, 14, 1520. https://doi.org/10.3390/pharmaceutics14071520
Deguchi S, Kadowaki R, Otake H, Taga A, Nakazawa Y, Misra M, Yamamoto N, Sasaki H, Nagai N. Combination of Lanosterol and Nilvadipine Nanosuspensions Rescues Lens Opacification in Selenite-Induced Cataractic Rats. Pharmaceutics. 2022; 14(7):1520. https://doi.org/10.3390/pharmaceutics14071520
Chicago/Turabian StyleDeguchi, Saori, Reita Kadowaki, Hiroko Otake, Atsushi Taga, Yosuke Nakazawa, Manju Misra, Naoki Yamamoto, Hiroshi Sasaki, and Noriaki Nagai. 2022. "Combination of Lanosterol and Nilvadipine Nanosuspensions Rescues Lens Opacification in Selenite-Induced Cataractic Rats" Pharmaceutics 14, no. 7: 1520. https://doi.org/10.3390/pharmaceutics14071520
APA StyleDeguchi, S., Kadowaki, R., Otake, H., Taga, A., Nakazawa, Y., Misra, M., Yamamoto, N., Sasaki, H., & Nagai, N. (2022). Combination of Lanosterol and Nilvadipine Nanosuspensions Rescues Lens Opacification in Selenite-Induced Cataractic Rats. Pharmaceutics, 14(7), 1520. https://doi.org/10.3390/pharmaceutics14071520