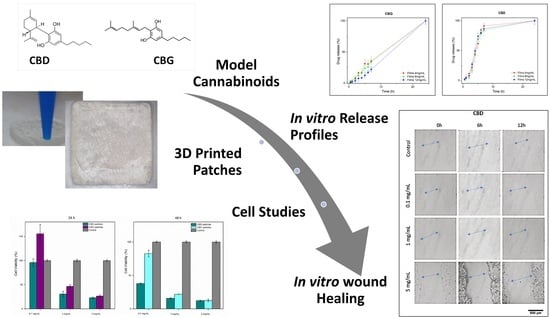
Fabrication and Preliminary In Vitro Evaluation of 3D-Printed Alginate Films with Cannabidiol (CBD) and Cannabigerol (CBG) Nanoparticles for Potential Wound-Healing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CBD and CBG Solid Suspensions
2.3. Development of the 3D-Printed Films
2.4. Differential Scanning Calorimetry (DSC)
2.5. Thermo-Gravimetric Analysis (TGA)
2.6. Fourier-Transform Infra-Red (FTIR)
2.7. Particle Size and Polydispersity Index (PDI)
2.8. Preliminary Characterization of the Films
2.9. Water Retention Capacity and Porosity
2.10. Water Loss Rate
2.11. Optical Microscopy
2.12. Mechanical Tests
2.13. 3D-Printing Shape Fidelity Assessment
2.14. Quantitative Analysis of Cannabinoids
2.15. In Vitro Release Study of Cannabinoids from Loaded Films
2.16. Entrapment Efficiency and Drug Loading
2.17. Cell Culture
2.18. Cell Viability—MTT Assay
2.19. Cell Scratch Assay (Wound Healing)
2.20. Antibacterial Activity In Vitro
2.21. Statistical Analysis
3. Results
3.1. Development of Cannabinoid Nanoparticles
3.2. Evaluation of 3D Printed Films
3.2.1. pH Determination
3.2.2. Film Water Loss Rate
3.2.3. Film Surface Observation
3.2.4. Water Retention Capacity and Porosity
3.2.5. Mechanical Tests
3.3. Fourier-Transform Infra-Red (FTIR)
3.4. Differential Scanning Calorimetry (DSC)
3.5. Thermogravimetric Analysis (TGA)
3.6. In Vitro Release of CBD and CBG from the Films
3.7. Drug Loading and Entrapment Efficiency of Nanoparticles
3.8. Cell Viability—MTT Assay
3.9. Cell Scratch Assay (In Vitro Wound Healing)
3.10. Evaluation of In Vitro Antibacterial Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health Economic Burden That Wounds Impose on the National Health Service in the UK. BMJ Open 2015, 5, e009283. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Acevedo, M.C.; Mendoza-Flores, R.A.; Del Prado-Audelo, M.L.; Urbán-Morlán, Z.; Giraldo-Gomez, D.M.; Magaña, J.J.; González-Torres, M.; Reyes-Hernández, O.D.; Figueroa-González, G.; Caballero-Florán, I.H.; et al. Development and Evaluation of Alginate Membranes with Curcumin-Loaded Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics 2019, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Adis Medical Writers. Select Appropriate Wound Dressings by Matching the Properties of the Dressing to the Type of Wound. Drugs Ther. Perspect. 2014, 30, 213–217. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound Dressings—A Review. BioMed 2015, 5, 22. [Google Scholar] [CrossRef]
- Mi, F.-L.; Wu, Y.-B.; Shyu, S.-S.; Chao, A.-C.; Lai, J.-Y.; Su, C.-C. Asymmetric Chitosan Membranes Prepared by Dry/Wet Phase Separation: A New Type of Wound Dressing for Controlled Antibacterial Release. J. Membr. Sci. 2003, 212, 237–254. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Eleftheriadis, G.K.; Karavasili, C.; Fatouros, D.G. Development of Bio-Active Patches Based on Pectin for the Treatment of Ulcers and Wounds Using 3D-Bioprinting Technology. Pharmaceutics 2020, 12, 56. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Monou, P.-K.; Louka, A.; Papaefstathiou, E.; Eleftheriadis, G.K.; Fatouros, D.G. Development of Food Grade 3D Printable Ink Based on Pectin Containing Cannabidiol/Cyclodextrin Inclusion Complexes. Drug Dev. Ind. Pharm. 2020, 46, 1569–1577. [Google Scholar] [CrossRef]
- Franco, P.; Pessolano, E.; Belvedere, R.; Petrella, A.; De Marco, I. Supercritical Impregnation of Mesoglycan into Calcium Alginate Aerogel for Wound Healing. J. Supercrit. Fluids 2020, 157, 104711. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Braga, M.E.M.; Seabra, I.J.; Ferreira, P.; Gil, M.H.; de Sousa, H.C. Development of Natural-Based Wound Dressings Impregnated with Bioactive Compounds and Using Supercritical Carbon Dioxide. Int. J. Pharm. 2011, 408, 9–19. [Google Scholar] [CrossRef]
- Chen, K.; Pan, H.; Ji, D.; Li, Y.; Duan, H.; Pan, W. Curcumin-Loaded Sandwich-like Nanofibrous Membrane Prepared by Electrospinning Technology as Wound Dressing for Accelerate Wound Healing. Mater. Sci. Eng. C 2021, 127, 112245. [Google Scholar] [CrossRef]
- Teoh, J.H.; Tay, S.M.; Fuh, J.; Wang, C.-H. Fabricating Scalable, Personalized Wound Dressings with Customizable Drug Loadings via 3D Printing. J. Control. Release 2022, 341, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Štukelj, R.; Benčina, M.; Fanetti, M.; Valant, M.; Drab, M.; Kralj-iglic, V. Synthesis of Stable Cannabidiol (CBD) Nanoparticles in Suspension. Mater. Tehnol. 2019, 53, 543–549. [Google Scholar] [CrossRef]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Treister-Goltzman, Y.; Freud, T.; Press, Y.; Peleg, R. Trends in Publications on Medical Cannabis from the Year 2000. Popul. Health Manag. 2019, 22, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.; Filloux, F.; et al. Cannabidiol in Patients with Treatment-Resistant Epilepsy: An Open-Label Interventional Trial. Lancet Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef]
- Zuardi, A.W. Cannabidiol: From an Inactive Cannabinoid to a Drug with Wide Spectrum of Action. Braz. J. Psychiatry 2008, 30, 271–280. [Google Scholar] [CrossRef]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef]
- Dinu, A.R.; Rogobete, A.F.; Bratu, T.; Popovici, S.E.; Bedreag, O.H.; Papurica, M.; Bratu, L.M.; Sandesc, D. Cannabis Sativa Revisited—Crosstalk between MicroRNA Expression, Inflammation, Oxidative Stress, and Endocannabinoid Response System in Critically Ill Patients with Sepsis. Cells 2020, 9, 307. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the Art and New Challenges for Therapeutic Applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- del Río, C.; Millán, E.; García, V.; Appendino, G.; DeMesa, J.; Muñoz, E. The Endocannabinoid System of the Skin. A Potential Approach for the Treatment of Skin Disorders. Biochem. Pharmacol. 2018, 157, 122–133. [Google Scholar] [CrossRef]
- Sheriff, T.; Lin, M.J.; Dubin, D.; Khorasani, H. The Potential Role of Cannabinoids in Dermatology. J. Dermatol. Treat. 2020, 31, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S. Cannabidiol (CBD) and Its Analogs: A Review of Their Effects on Inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as Novel Anti-Inflammatory Drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis Sativa L. Extract and Cannabidiol Inhibit in Vitro Mediators of Skin Inflammation and Wound Injury. Phytother. Res. 2019, 33, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, M.A.; Sivamani, R.; Lev-Tov, H. The Therapeutic Potential of Cannabinoids for Integumentary Wound Management. Exp. Dermatol. 2021, 30, 201–211. [Google Scholar] [CrossRef]
- Namdar, D.; Koltai, H. Medical Cannabis for the Treatment of Inflammation. 2018. Available online: https://journals.sagepub.com/doi/abs/10.1177/1934578X1801300304 (accessed on 23 June 2022).
- Hampson, A.J.; Grimaldi, M.; Lolic, M.; Wink, D.; Rosenthal, R.; Axelrod, J. Neuroprotective Antioxidants from Marijuanaa. Ann. N. Y. Acad. Sci. 2000, 899, 274–282. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Slade, D. Chemical Constituents of Marijuana: The Complex Mixture of Natural Cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- FDA Approves New Indication for Drug Containing an Active Ingredient Derived from Cannabis to Treat Seizures in Rare Genetic Disease. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-indication-drug-containing-active-ingredient-derived-cannabis-treat-seizures-rare (accessed on 23 June 2022).
- Stinchcomb, A.L.; Valiveti, S.; Hammell, D.C.; Ramsey, D.R. Human Skin Permeation of Delta8-Tetrahydrocannabinol, Cannabidiol and Cannabinol. J. Pharm. Pharmacol. 2004, 56, 291–297. [Google Scholar] [CrossRef]
- Chen, H.; Khemtong, C.; Yang, X.; Chang, X.; Gao, J. Nanonization Strategies for Poorly Water-Soluble Drugs. Drug Discov. Today 2011, 16, 354–360. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric Micelles and Alternative Nanonized Delivery Vehicles for Poorly Soluble Drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® Block Copolymers as Novel Polymer Therapeutics for Drug and Gene Delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef]
- Hou, J.; Wang, J.; Sun, E.; Yang, L.; Yan, H.-M.; Jia, X.-B.; Zhang, Z.-H. Preparation and Evaluation of Icariside II-Loaded Binary Mixed Micelles Using Solutol HS15 and Pluronic F127 as Carriers. Drug Deliv. 2016, 23, 3248–3256. [Google Scholar] [CrossRef]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Eleftheriadis, G.K.; Kantarelis, E.; Monou, P.K.; Andriotis, E.G.; Bouropoulos, N.; Tzimtzimis, E.K.; Tzetzis, D.; Rantanen, J.; Fatouros, D.G. Automated Digital Design for 3D-Printed Individualized Therapies. Int. J. Pharm. 2021, 599, 120437. [Google Scholar] [CrossRef]
- Karavasili, C.; Eleftheriadis, G.K.; Gioumouxouzis, C.; Andriotis, E.G.; Fatouros, D.G. Mucosal Drug Delivery and 3D Printing Technologies: A Focus on Special Patient Populations. Adv. Drug Deliv. Rev. 2021, 176, 113858. [Google Scholar] [CrossRef]
- Andreadis, I.I.; Gioumouxouzis, C.I.; Eleftheriadis, G.K.; Fatouros, D.G. The Advent of a New Era in Digital Healthcare: A Role for 3D Printing Technologies in Drug Manufacturing? Pharmaceutics 2022, 14, 609. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Boetker, J.; Rantanen, J.; Jacobsen, J.; Vizirianakis, I.S.; Fatouros, D.G. Fabrication of Mucoadhesive Buccal Films for Local Administration of Ketoprofen and Lidocaine Hydrochloride by Combining Fused Deposition Modeling and Inkjet Printing. J. Pharm. Sci. 2020, 109, 2757–2766. [Google Scholar] [CrossRef]
- Karavasili, C.; Tsongas, K.; Andreadis, I.I.; Andriotis, E.G.; Papachristou, E.T.; Papi, R.M.; Tzetzis, D.; Fatouros, D.G. Physico-Mechanical and Finite Element Analysis Evaluation of 3D Printable Alginate-Methylcellulose Inks for Wound Healing Applications. Carbohydr. Polym. 2020, 247, 116666. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Radebaugh, G.W.; Murtha, J.L.; Julian, T.N.; Bondi, J.N. Methods for Evaluating the Puncture and Shear Properties of Pharmaceutical Polymeric Films. Int. J. Pharm. 1988, 45, 39–46. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Chachlioutaki, K.; Monou, P.K.; Bouropoulos, N.; Tzetzis, D.; Barmpalexis, P.; Chang, M.-W.; Ahmad, Z.; Fatouros, D.G. Development of Water-Soluble Electrospun Fibers for the Oral Delivery of Cannabinoids. AAPS PharmSciTech 2021, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, A.E.; Salah, S.; Amer, M.S.; Elkasabgy, N.A. 3D Nanocomposite Alginate Hydrogel Loaded with Pitavastatin Nanovesicles as a Functional Wound Dressing with Controlled Drug Release; Preparation, in-Vitro and in-Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2022, 71, 103292. [Google Scholar] [CrossRef]
- Shu, G.; Xu, D.; Zhang, W.; Zhao, X.; Li, H.; Xu, F.; Yin, L.; Peng, X.; Fu, H.; Chang, L.-J.; et al. Preparation of Shikonin Liposome and Evaluation of Its in Vitro Antibacterial and in Vivo Infected Wound Healing Activity. Phytomedicine 2022, 99, 154035. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, M.; Zhang, Y.; Zeng, J.; Omari-Siaw, E.; Yu, J.; Xu, X. In Vitro Release and Bioavailability of Silybin from Micelle-Templated Porous Calcium Phosphate Microparticles. AAPS PharmSciTech 2016, 17, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Alghurabi, H.; Tagami, T.; Ogawa, K.; Ozeki, T. Preparation, Characterization and In Vitro Evaluation of Eudragit S100-Coated Bile Salt-Containing Liposomes for Oral Colonic Delivery of Budesonide. Polymers 2022, 14, 2693. [Google Scholar] [CrossRef]
- Karas, J.A.; Wong, L.J.M.; Paulin, O.K.A.; Mazeh, A.C.; Hussein, M.H.; Li, J.; Velkov, T. The Antimicrobial Activity of Cannabinoids. Antibiotics 2020, 9, 406. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Papi, R.M.; Paraskevopoulou, A.; Achilias, D.S. Synthesis of D-Limonene Loaded Polymeric Nanoparticles with Enhanced Antimicrobial Properties for Potential Application in Food Packaging. Nanomaterials 2021, 11, 191. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-Box Model-Based Gelation of Alginate and Pectin: A Review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Badita, C.; Aranghel, D.; Burducea, C.; Mereuță, P. Characterization of Sodium Alginate Based Films. Available online: https://www.semanticscholar.org/paper/characterization-of-sodium-alginate-based-films-badita-aranghel/8308f9f4934bb7d5594bbe4b71fbfb004fa0a836 (accessed on 23 June 2022).
- Uzun, M.; Anand, S.C.; Shah, T. The Effect of Wound Dressings on the PH Stability of Fluids. J. Wound Care 2012, 21, 88–95. [Google Scholar] [CrossRef]
- Matarazzo, A.P.; Elisei, L.M.S.; Carvalho, F.C.; Bonfílio, R.; Ruela, A.L.M.; Galdino, G.; Pereira, G.R. Mucoadhesive Nanostructured Lipid Carriers as a Cannabidiol Nasal Delivery System for the Treatment of Neuropathic Pain. Eur. J. Pharm. Sci. 2021, 159, 105698. [Google Scholar] [CrossRef]
- Rhim, J.-W. Physical and Mechanical Properties of Water Resistant Sodium Alginate Films. LWT-Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Olmos-Juste, R.; Alonso-Lerma, B.; Pérez-Jiménez, R.; Gabilondo, N.; Eceiza, A. 3D Printed Alginate-Cellulose Nanofibers Based Patches for Local Curcumin Administration. Carbohydr. Polym. 2021, 264, 118026. [Google Scholar] [CrossRef]
- Erzengin, S.; Guler, E.; Eser, E.; Polat, E.B.; Gunduz, O.; Cam, M.E. In Vitro and In Vivo Evaluation of 3D Printed Sodium Alginate/Polyethylene Glycol Scaffolds for Sublingual Delivery of Insulin: Preparation, Characterization, and Pharmacokinetics. Int. J. Biol. Macromol. 2022, 204, 429–440. [Google Scholar] [CrossRef]
- McClements, D.J. Designing Biopolymer Microgels to Encapsulate, Protect and Deliver Bioactive Components: Physicochemical Aspects. Adv. Colloid Interface Sci. 2017, 240, 31–59. [Google Scholar] [CrossRef]
- Singh, B.; Pal, L. Radiation Crosslinking Polymerization of Sterculia Polysaccharide–PVA–PVP for Making Hydrogel Wound Dressings. Int. J. Biol. Macromol. 2011, 48, 501–510. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-Based Composite Materials for Wound Dressing Application:A Mini Review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and Characterization of Films Based on Alginate and Aloe Vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Butt, A.M.; Amin, M.C.I.M.; Katas, H.; Sarisuta, N.; Witoonsaridsilp, W.; Benjakul, R. In Vitro Characterization of Pluronic F127 and D--Tocopheryl Polyethylene Glycol 1000 Succinate Mixed Micelles as Nanocarriers for Targeted Anticancer-Drug Delivery. J. Nanomater. 2012, 2012, e916573. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Ren, D.-Y.; Feng, Z.-X.; Zhang, L.-Y.; Zhong, Y.-F.; Jin, M.-Y.; Xu, F.-W.; Feng, C.-Y.; Du, Y.-Z.; et al. Mussel-Inspired Collagen-Hyaluronic Acid Composite Scaffold with Excellent Antioxidant Properties and Sustained Release of a Growth Factor for Enhancing Diabetic Wound Healing. Mater. Today Bio 2022, 15, 100320. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.; Wat, E.; Kan, C.; Leung, P.-C.; Wang, W. Drug Delivery System of Dual-Responsive PF127 Hydrogel with Polysaccharide-Based Nano-Conjugate for Textile-Based Transdermal Therapy. Carbohydr. Polym. 2020, 236, 116074. [Google Scholar] [CrossRef]
- Klein, M.; de Quadros De Bortolli, J.; Guimarães, F.S.; Salum, F.G.; Cherubini, K.; de Figueiredo, M.A.Z. Effects of Cannabidiol, a Cannabis Sativa Constituent, on Oral Wound Healing Process in Rats: Clinical and Histological Evaluation. Phytother. Res. 2018, 32, 2275–2281. [Google Scholar] [CrossRef]
- Kongkadee, K.; Wisuitiprot, W.; Ingkaninan, K.; Waranuch, N. Anti-Inflammation and Gingival Wound Healing Activities of Cannabis Sativa L. Subsp. Sativa (Hemp) Extract and Cannabidiol: An in Vitro Study. Arch. Oral Biol. 2022, 140, 105464. [Google Scholar] [CrossRef]
- Perez, E.; Fernandez, J.R.; Fitzgerald, C.; Rouzard, K.; Tamura, M.; Savile, C. In Vitro and Clinical Evaluation of Cannabigerol (CBG) Produced via Yeast Biosynthesis: A Cannabinoid with a Broad Range of Anti-Inflammatory and Skin Health-Boosting Properties. Molecules 2022, 27, 491. [Google Scholar] [CrossRef]
- Essaghraoui, A.; Belfkira, A.; Hamdaoui, B.; Nunes, C.; Lima, S.A.C.; Reis, S. Improved Dermal Delivery of Cyclosporine A Loaded in Solid Lipid Nanoparticles. Nanomaterials 2019, 9, 1204. [Google Scholar] [CrossRef]













| Formulation Code | Component Concentration | ||
|---|---|---|---|
| SA (% w/v) | Calcium Chloride (% w/v) | Cannabinoid (mg/mL) | |
| M1 | 6 | 0.4 | 4 |
| M2 | 6 | 0.4 | 8 |
| M3 | 6 | 0.4 | 12 |
| Preparation Method (Cannabinoid:PF127 1:1) | ||||
| CBD | CBG | |||
| PdI | Z-Average Size (nm) | PdI | Z-Average Size (nm) | |
| Method A | 0.203 | 166 | 0.202 | 188 |
| Method B | 0.272 | 127 | 0.247 | 195 |
| Cannabinoid:PF127 Ratio Comparison (Method A) | ||||
| CBD | CBG | |||
| Cannabinoid Ratio (% w/v) | PdI | Z-Average Size (nm) | PdI | Z-Average Size (nm) |
| 100 | 0.634 | 663 | 0.539 | 1270 |
| 90 | 1.000 | 252 | 1.000 | 845 |
| 80 | 0.511 | 410 | 0.649 | 4550 |
| 70 | 0.846 | 120 | 0.724 | 1200 |
| 60 | 0.554 | 364 | 0.635 | 4300 |
| 50 | 0.203 | 166 | 0.485 | 282 |
| 40 | 0.406 | 154 | 0.850 | 265 |
| 30 | 0.380 | 189 | 0.651 | 182 |
| 20 | 0.281 | 144 | 0.603 | 189 |
| 10 | 0.254 | 65.5 | 0.118 | 35.2 |
| 0 | 1.000 | 148 | 0.110 | 68.7 |
| Dilution in Water Comparison (Method B) | ||||
| CBD | CBG | |||
| Cannabinoid Ratio (% w/v) | PdI | Z-Average Size (nm) | PdI | Z-Average Size (nm) |
| 0.4 | 0.355 | 186 | 0.374 | 346 |
| 0.8 | 0.316 | 221 | 0.265 | 264 |
| 4 | 0.247 | 190 | 0.185 | 192 |
| Value | STD | |
|---|---|---|
| Thickness | 0.8 mm | ±0.002 |
| Weight | 0.73 g | ±0.150 |
| Shape Fidelity Assessment | 7.51% | ±0.150 |
| Porosity | 11.00 | ±0.002 |
| Formulations | Growth Inhibition Zone (mm) | ||
|---|---|---|---|
| E. coli | S. aureus | Bacillus spp. | |
| Control | - | - | - |
| CBD (5 mg/mL) | - | 12.7 ± 1.2 | 11.7 ± 0.6 |
| CBG (5 mg/mL) | 7 ± 0.1 | 13.3 ± 1.5 | 10.3 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monou, P.K.; Mamaligka, A.M.; Tzimtzimis, E.K.; Tzetzis, D.; Vergkizi-Nikolakaki, S.; Vizirianakis, I.S.; Andriotis, E.G.; Eleftheriadis, G.K.; Fatouros, D.G. Fabrication and Preliminary In Vitro Evaluation of 3D-Printed Alginate Films with Cannabidiol (CBD) and Cannabigerol (CBG) Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics 2022, 14, 1637. https://doi.org/10.3390/pharmaceutics14081637
Monou PK, Mamaligka AM, Tzimtzimis EK, Tzetzis D, Vergkizi-Nikolakaki S, Vizirianakis IS, Andriotis EG, Eleftheriadis GK, Fatouros DG. Fabrication and Preliminary In Vitro Evaluation of 3D-Printed Alginate Films with Cannabidiol (CBD) and Cannabigerol (CBG) Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics. 2022; 14(8):1637. https://doi.org/10.3390/pharmaceutics14081637
Chicago/Turabian StyleMonou, Paraskevi Kyriaki, Anastasia Maria Mamaligka, Emmanuil K. Tzimtzimis, Dimitrios Tzetzis, Souzan Vergkizi-Nikolakaki, Ioannis S. Vizirianakis, Eleftherios G. Andriotis, Georgios K. Eleftheriadis, and Dimitrios G. Fatouros. 2022. "Fabrication and Preliminary In Vitro Evaluation of 3D-Printed Alginate Films with Cannabidiol (CBD) and Cannabigerol (CBG) Nanoparticles for Potential Wound-Healing Applications" Pharmaceutics 14, no. 8: 1637. https://doi.org/10.3390/pharmaceutics14081637
APA StyleMonou, P. K., Mamaligka, A. M., Tzimtzimis, E. K., Tzetzis, D., Vergkizi-Nikolakaki, S., Vizirianakis, I. S., Andriotis, E. G., Eleftheriadis, G. K., & Fatouros, D. G. (2022). Fabrication and Preliminary In Vitro Evaluation of 3D-Printed Alginate Films with Cannabidiol (CBD) and Cannabigerol (CBG) Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics, 14(8), 1637. https://doi.org/10.3390/pharmaceutics14081637