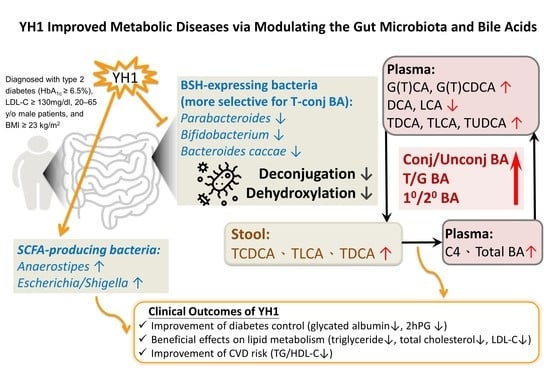
Gut Microbiota and Bile Acids Mediate the Clinical Benefits of YH1 in Male Patients with Type 2 Diabetes Mellitus: A Pilot Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Microbiome, Metabolomic, and Biochemical Measures
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Microbiota Profiles
3.3. Changes in Plasma and Fecal Bile Acid Profiles, Plasma C4, and FGF19 Levels
3.4. Changes in Clinical Parameters and the Correlation between YH1-Responsive Microbiota and BA or Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tong, X.L.; Dong, L.; Chen, L.; Zhen, Z. Treatment of diabetes using traditional Chinese medicine: Past, present and future. Am. J. Chin. Med. 2012, 40, 877–886. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Tian, J.X.; Lian, F.M.; Li, M.; Liu, W.K.; Zhen, Z.; Liao, J.Q.; Tong, X.L. Therapeutic mechanisms of traditional Chinese medicine to improve metabolic diseases via the gut microbiota. Biomed. Pharmacother. 2021, 133, 110857. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Chen, S.T.; Liu, F.H.; Hsieh, S.H.; Lin, C.H.; Liou, M.J.; Wang, C.C.; Huang, C.H.; Liu, G.H.; Lin, J.R.; et al. The efficacy and safety of concentrated herbal extract granules, YH1, as an add-on medication in poorly controlled type 2 diabetes: A randomized, double-blind, placebo-controlled pilot trial. PLoS ONE 2019, 14, e0221199. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ye, J.; Jia, W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm. Sin. B 2012, 2, 327–334. [Google Scholar] [CrossRef]
- Habtemariam, S. The Quest to Enhance the Efficacy of Berberine for Type-2 Diabetes and Associated Diseases: Physicochemical Modification Approaches. Biomedicines 2020, 8, 90. [Google Scholar] [CrossRef]
- He, K.; Hu, Y.; Ma, H.; Zou, Z.; Xiao, Y.; Yang, Y.; Feng, M.; Li, X.; Ye, X. Rhizoma coptidis alkaloids alleviate hyperlipidemia in B6 mice by modulating gut microbiota and bile acid pathways. Biochim. Biophys. Acta 2016, 1862, 1696–1709. [Google Scholar] [CrossRef]
- Tian, Y.; Cai, J.; Gui, W.; Nichols, R.G.; Koo, I.; Zhang, J.; Anitha, M.; Patterson, A.D. Berberine Directly Affects the Gut Microbiota to Promote Intestinal Farnesoid X Receptor Activation. Drug Metab. Dispos. 2019, 47, 86–93. [Google Scholar] [CrossRef]
- Wolf, P.G.; Devendran, S.; Doden, H.L.; Ly, L.K.; Moore, T.; Takei, H.; Nittono, H.; Murai, T.; Kurosawa, T.; Chlipala, G.E.; et al. Berberine alters gut microbial function through modulation of bile acids. BMC Microbiol. 2021, 21, 24. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Ren, H.; Wang, S.; Zhong, H.; Zhao, X.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat. Commun. 2020, 11, 5015. [Google Scholar] [CrossRef]
- Ramírez-Pérez, O.; Cruz-Ramón, V.; Chinchilla-López, P.; Méndez-Sánchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16 (Suppl. S1), S21–S26. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, J.M.; Chiang, J.Y.L. Understanding Bile Acid Signaling in Diabetes: From Pathophysiology to Therapeutic Targets. Diabetes Metab. J. 2019, 43, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Panzitt, K.; Zollner, G.; Marschall, H.U.; Wagner, M. Recent advances on FXR-targeting therapeutics. Mol. Cell. Endocrinol. 2022, 552, 111678. [Google Scholar] [CrossRef]
- Song, Z.; Cai, Y.; Lao, X.; Wang, X.; Lin, X.; Cui, Y.; Kalavagunta, P.K.; Liao, J.; Jin, L.; Shang, J.; et al. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome 2019, 7, 9. [Google Scholar] [CrossRef]
- Duboc, H.; Taché, Y.; Hofmann, A.F. The bile acid TGR5 membrane receptor: From basic research to clinical application. Dig. Liver Dis. 2014, 46, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Sepe, V.; Festa, C.; Renga, B.; Carino, A.; Cipriani, S.; Finamore, C.; Masullo, D.; Del Gaudio, F.; Monti, M.C.; Fiorucci, S.; et al. Insights on FXR selective modulation. Speculation on bile acid chemical space in the discovery of potent and selective agonists. Sci. Rep. 2016, 6, 19008. [Google Scholar] [CrossRef]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Wicksteed, B.; Schiltz, G.E.; Gilchrist, A.; Layden, B.T. SCFA Receptors in Pancreatic Beta Cells: Novel Diabetes Targets? Trends Endocrinol. Metab. 2016, 27, 653–664. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, L.; Liu, W.; Zou, B.; Cai, Y.; Liu, D.; Xiao, D.; Chen, J.; Li, P.; Zhong, Y.; et al. Shen-Ling-Bai-Zhu-San alleviates functional dyspepsia in rats and modulates the composition of the gut microbiota. Nutr. Res. 2019, 71, 89–99. [Google Scholar] [CrossRef]
- Lv, W.J.; Liu, C.; Li, Y.F.; Chen, W.Q.; Li, Z.Q.; Li, Y.; Xiong, Y.; Chao, L.M.; Xu, X.L.; Guo, S.N. Systems Pharmacology and Microbiome Dissection of Shen Ling Bai Zhu San Reveal Multiscale Treatment Strategy for IBD. Oxid. Med. Cell. Longev. 2019, 2019, 8194804. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shou, J.W.; Li, X.Y.; Zhao, Z.X.; Fu, J.; He, C.Y.; Feng, R.; Ma, C.; Wen, B.Y.; Guo, F.; et al. Berberine-induced bioactive metabolites of the gut microbiota improve energy metabolism. Metabolism 2017, 70, 72–84. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef]
- Yang, C.Y.; Yeh, Y.M.; Yu, H.Y.; Chin, C.Y.; Hsu, C.W.; Liu, H.; Huang, P.J.; Hu, S.N.; Liao, C.T.; Chang, K.P.; et al. Oral Microbiota Community Dynamics Associated with Oral Squamous Cell Carcinoma Staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef]
- Chiu, S.F.; Huang, P.J.; Cheng, W.H.; Huang, C.Y.; Chu, L.J.; Lee, C.C.; Lin, H.C.; Chen, L.C.; Lin, W.N.; Tsao, C.H.; et al. Vaginal Microbiota of the Sexually Transmitted Infections Caused by Chlamydia trachomatis and Trichomonas vaginalis in Women with Vaginitis in Taiwan. Microorganisms 2021, 9, 1864. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.C. SINTAX: A simple non-Bayesian taxonomy classifier for 16S and ITS sequences. bioRxiv 2016. bioRxiv:074161. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. eBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Sun, R.; Yang, N.; Kong, B.; Cao, B.; Feng, D.; Yu, X.; Ge, C.; Huang, J.; Shen, J.; Wang, P.; et al. Orally Administered Berberine Modulates Hepatic Lipid Metabolism by Altering Microbial Bile Acid Metabolism and the Intestinal FXR Signaling Pathway. Mol. Pharmacol. 2017, 91, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef]
- Yao, L.; Seaton, S.C.; Ndousse-Fetter, S.; Adhikari, A.A.; DiBenedetto, N.; Mina, A.I.; Banks, A.S.; Bry, L.; Devlin, A.S. A selective gut bacterial bile salt hydrolase alters host metabolism. eLife 2018, 7, e37182. [Google Scholar] [CrossRef]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef]
- Porez, G.; Prawitt, J.; Gross, B.; Staels, B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J. Lipid Res. 2012, 53, 1723–1737. [Google Scholar] [CrossRef]
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.e1673. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K.; Heidari, B.; Bakhtiari, A. Triglyceride to high-density lipoprotein cholesterol and low-density lipoprotein cholestrol to high-density lipoprotein cholesterol ratios are predictors of cardiovascular risk in Iranian adults: Evidence from a population-based cross-sectional study. Casp. J. Intern. Med. 2020, 11, 53–61. [Google Scholar] [CrossRef]
- Vega, G.L.; Barlow, C.E.; Grundy, S.M.; Leonard, D.; DeFina, L.F. Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J. Investig. Med. 2014, 62, 345–349. [Google Scholar] [CrossRef]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Kurl, S.; Laukkanen, J.A.; Niskanen, L.; Laaksonen, D.; Sivenius, J.; Nyyssönen, K.; Salonen, J.T. Metabolic syndrome and the risk of stroke in middle-aged men. Stroke 2006, 37, 806–811. [Google Scholar] [CrossRef] [Green Version]




| Pre-Treatment (n = 10) | Post-Treatment (n = 10) | p Value | |
|---|---|---|---|
| Median (Min, Max) | Median (Min, Max) | ||
| Demographic characteristics | |||
| Age (years) | 48 (38, 57) | ||
| Gender, Male/Female | 10/0 | ||
| Duration of DM (years) | 2.0 (0.5, 14.0) | ||
| Anthropometric characteristics and vital signs | |||
| Weight (kg) | 78.8 (68.4, 102.6) | 79.5 (68.2, 101.8) | 0.76 |
| BMI (kg/m2) | 27.2 (24.8, 34.2) | 27.4 (24.7, 33.3) | 0.77 |
| SBP (mmHg) | 133.0 (104.0, 153.0) | 128.5 (103.0, 150.0) | 0.42 |
| DBP (mmHg) | 86.0 (51.0, 101.0) | 77.0 (62.0, 104.0) | 0.17 |
| HR (beat/min) | 76.0 (60.0, 89.0) | 73.0 (56.0, 80.0) | 0.18 |
| Laboratory data | |||
| Glycated albumin (%) | 16.8 (14.0, 32.1) | 15.3 (12.8, 29.8) | 0.005 ** |
| FPG (mg/dL) | 129.5 (81.0, 277.0) | 130.0 (86.0, 255.0) | 0.21 |
| 2hPG (mg/dL) | 158.0 (111.0, 335.0) | 117.5 (98.0, 304.0) | 0.007 ** |
| ALT (U/L) | 49.5 (21.0, 69.0) | 39.5 (12.0, 74.0) | 0.07 |
| Cr (mg/dL) | 0.9 (0.5, 0.9) | 0.7 (0.6, 1.0) | 0.10 |
| Total cholesterol(mg/dL) | 231.5 (212.0, 262.0) | 193.0 (141.0, 223.0) | 0.007 ** |
| HDL-C (mg/dL) | 43.5 (34.0, 61.0) | 40.0 (29.0, 50.0) | 0.02 * |
| LDL-C (mg/dL) | 150.0 (139.0, 196.0) | 134.5 (88.0, 150.0) | 0.007 ** |
| Triglycerides (mg/dL) | 194.0 (74.0, 497.0) | 103.5 (58.0, 211.0) | 0.005 ** |
| Triglycerides/HDL-C | 4.4 (1.2, 11.8) | 2.7 (1.2, 4.9) | 0.009 ** |
| Fasting insulin (μU/mL) | 10.2 (5.7, 20.1) | 11.0 (5.3, 26.6) | 0.14 |
| HOMA-IR | 3.8 (2.1, 7.7) | 3.5 (2.0, 10.1) | 0.24 |
| HOMA-β | 55.3 (13.0, 402.0) | 81.9 (16.9, 275.1) | 0.14 |
| CRP (mg/L) | 2.5 (0.4, 4.8) # | 1.9 (0.6, 13.5) # | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-H.; Wu, Y.-H.; Tang, H.-Y.; Chen, S.-T.; Wang, C.-C.; Ho, W.-J.; Lin, Y.-H.; Liu, G.-H.; Lin, P.-Y.; Lo, C.-J.; et al. Gut Microbiota and Bile Acids Mediate the Clinical Benefits of YH1 in Male Patients with Type 2 Diabetes Mellitus: A Pilot Observational Study. Pharmaceutics 2022, 14, 1857. https://doi.org/10.3390/pharmaceutics14091857
Huang Y-H, Wu Y-H, Tang H-Y, Chen S-T, Wang C-C, Ho W-J, Lin Y-H, Liu G-H, Lin P-Y, Lo C-J, et al. Gut Microbiota and Bile Acids Mediate the Clinical Benefits of YH1 in Male Patients with Type 2 Diabetes Mellitus: A Pilot Observational Study. Pharmaceutics. 2022; 14(9):1857. https://doi.org/10.3390/pharmaceutics14091857
Chicago/Turabian StyleHuang, Yueh-Hsiang, Yi-Hong Wu, Hsiang-Yu Tang, Szu-Tah Chen, Chih-Ching Wang, Wan-Jing Ho, Yi-Hsuan Lin, Geng-Hao Liu, Pei-Yeh Lin, Chi-Jen Lo, and et al. 2022. "Gut Microbiota and Bile Acids Mediate the Clinical Benefits of YH1 in Male Patients with Type 2 Diabetes Mellitus: A Pilot Observational Study" Pharmaceutics 14, no. 9: 1857. https://doi.org/10.3390/pharmaceutics14091857
APA StyleHuang, Y. -H., Wu, Y. -H., Tang, H. -Y., Chen, S. -T., Wang, C. -C., Ho, W. -J., Lin, Y. -H., Liu, G. -H., Lin, P. -Y., Lo, C. -J., Yeh, Y. -M., & Cheng, M. -L. (2022). Gut Microbiota and Bile Acids Mediate the Clinical Benefits of YH1 in Male Patients with Type 2 Diabetes Mellitus: A Pilot Observational Study. Pharmaceutics, 14(9), 1857. https://doi.org/10.3390/pharmaceutics14091857