Punicalagin’s Protective Effects on Parkinson’s Progression in Socially Isolated and Socialized Rats: Insights into Multifaceted Pathway
Abstract
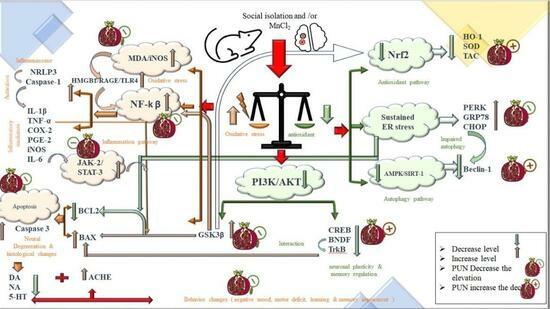
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and Chemicals
2.3. Experimental Design
2.4. Animal Housing Conditions
2.5. Behavioural Evaluations
2.5.1. Open Field Test (OFT)
2.5.2. Forced Swim Test (FST)
2.5.3. Y-Maze Test
2.6. Tissue Sample Collection and Preparation
2.7. Biochemical Assessments
2.7.1. Colormetric Assays in the Brain
2.7.2. Fluorometric Assays in the Brain
2.7.3. Enzyme Linked Immunosorbent Assay (ELISA) in Brain Tissues
2.7.4. Gene Expression Measurement by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Histopathological Evaluations
2.9. Statistical Analysis
3. Results
3.1. Impacts of PUN on Motor Functions in Open Field Test in SI and/or MnCl2 Intoxicated Rats
3.2. Impacts of PUN on Behavioural Parameters in Forced Swim Test (FST) and Y-Maze Test in SI and/or MnCl2-Intoxicated Rats
3.3. Impacts of PUN on Brain Monoamine Levels and ACHE Activity in SI and/or MnCl2-Intoxicated Rats
3.4. Impacts of PUN on Nrf2/HO-1 Pathway and Brain Oxidative Stress Biomarkers in SI and/or MnCl2-Intoxicated Rats
3.5. Impacts of PUN on Brain Inflammatory Biomarkers (NF-ᴋB, TNF-α, IL-1β, IL-6, COX-2 and PGE2) in SI and/or MnCl2-Intoxicated Rats
3.6. Impacts of PUN on HMGB1/RAGE/TLR4; NLRP3/Caspase-1 and JAK-2/STAT-3 Pathways in SI and/or MnCl2-Intoxicated Rats
3.7. Impacts of PUN on PI3K/AKT/GSK-3β/CREB Pathway in SI and/or MnCl2-Intoxicated Rats
3.8. Impacts of PUN on Endoplasmic Reticulum Stress Biomarkers (PERK, GRP78 and CHOP) and Apoptotic Biomarkers (Bcl-2, Bax and Caspase-3) in SI and/or MnCl2-Intoxicated Rats
3.9. Impacts of Punicalagin on AMPK/SIRT-1 Pathway in SI and/or MnCl2-Intoxicated Rats
3.10. Impacts of Punicalagin on Histopathological Alterations of Different Brain Regions in SI and/or MnCl2-Intoxicated Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Massano, J.; Bhatia, K.P. Clinical Approach to Parkinson’s Disease: Features, Diagnosis, and Principles of Management. Cold Spring Harb. Perspect. Med. 2012, 2, a008870. [Google Scholar] [CrossRef]
- Schulz-Schaeffer, W.J. The Synaptic Pathology of α-Synuclein Aggregation in Dementia with Lewy Bodies, Parkinson’s Disease and Parkinson’s Disease Dementia. Acta Neuropathol. 2010, 120, 131–143. [Google Scholar] [CrossRef]
- Baek, J.-H.; Mamula, D.; Tingstam, B.; Pereira, M.; He, Y.; Svenningsson, P. GRP78 Level Is Altered in the Brain, but Not in Plasma or Cerebrospinal Fluid in Parkinson’s Disease Patients. Front. Neurosci. 2019, 13, 697. [Google Scholar] [CrossRef]
- Esteves, A.R.; Cardoso, S.M. Differential Protein Expression in Diverse Brain Areas of Parkinson’s and Alzheimer’s Disease Patients. Sci. Rep. 2020, 10, 13149. [Google Scholar] [CrossRef]
- Vallée, A.; Vallée, J.-N.; Lecarpentier, Y. Parkinson’s Disease: Potential Actions of Lithium by Targeting the WNT/β-Catenin Pathway, Oxidative Stress, Inflammation and Glutamatergic Pathway. Cells 2021, 10, 230. [Google Scholar] [CrossRef]
- Huang, B.; Liu, J.; Meng, T.; Li, Y.; He, D.; Ran, X.; Chen, G.; Guo, W.; Kan, X.; Fu, S.; et al. Polydatin Prevents Lipopolysaccharide (LPS)-Induced Parkinson’s Disease via Regulation of the AKT/GSK3β-Nrf2/NF-ΚB Signaling Axis. Front. Immunol. 2018, 9, 2527. [Google Scholar] [CrossRef]
- Lotze, M.T.; Tracey, K.J. High-Mobility Group Box 1 Protein (HMGB1): Nuclear Weapon in the Immune Arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Afonina, I.S.; Zhong, Z.; Karin, M.; Beyaert, R. Limiting Inflammation—The Negative Regulation of NF-ΚB and the NLRP3 Inflammasome. Nat. Immunol. 2017, 18, 861–869. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, C.; Wang, K.; Liu, X.; Wang, H.; Che, Y.; Sun, F.; Zhou, X.; Zhao, X.; Wang, Q. Paraquat and Maneb Co-Exposure Induces Noradrenergic Locus Coeruleus Neurodegeneration through NADPH Oxidase-Mediated Microglial Activation. Toxicology 2017, 380, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Credle, J.J.; George, J.L.; Wills, J.; Duka, V.; Shah, K.; Lee, Y.-C.; Rodriguez, O.; Simkins, T.; Winter, M.; Moechars, D.; et al. GSK-3β Dysregulation Contributes to Parkinson’s-like Pathophysiology with Associated Region-Specific Phosphorylation and Accumulation of Tau and α-Synuclein. Cell Death Differ. 2015, 22, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, J.; Dhaliwal, N.; Akhtar, A.; Kuhad, A.; Chopra, K. Tetramethylpyrazine Attenuates Cognitive Impairment Via Suppressing Oxidative Stress, Neuroinflammation, and Apoptosis in Type 2 Diabetic Rats. Neurochem. Res. 2022, 47, 2431–2444. [Google Scholar] [CrossRef]
- Zahra, W.; Birla, H.; Singh, S.S.; Rathore, A.S.; Dilnashin, H.; Singh, R.; Keshri, P.K.; Singh, S.; Singh, S.P. Anti-Parkinsonian Effect of Mucuna Pruriens and Ursolic Acid on GSK3β/Calcium Signaling in Neuroprotection against Rotenone-Induced Parkinsonism. Phytomed. Plus 2022, 2, 100343. [Google Scholar] [CrossRef]
- Martínez, G.; Duran-Aniotz, C.; Cabral-Miranda, F.; Vivar, J.P.; Hetz, C. Endoplasmic Reticulum Proteostasis Impairment in Aging. Aging Cell 2017, 16, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The Unfolded Protein Response: Controlling Cell Fate Decisions under ER Stress and Beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Nakka, V.P.; Gusain, A.; Raghubir, R. Endoplasmic Reticulum Stress Plays Critical Role in Brain Damage After Cerebral Ischemia/Reperfusion in Rats. Neurotox. Res. 2010, 17, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Ghemrawi, R.; Khair, M. Endoplasmic Reticulum Stress and Unfolded Protein Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 6127. [Google Scholar] [CrossRef]
- Kudo, T.; Kanemoto, S.; Hara, H.; Morimoto, N.; Morihara, T.; Kimura, R.; Tabira, T.; Imaizumi, K.; Takeda, M. A Molecular Chaperone Inducer Protects Neurons from ER Stress. Cell Death Differ. 2008, 15, 364–375. [Google Scholar] [CrossRef]
- Ren, H.; Zhai, W.; Lu, X.; Wang, G. The Cross-Links of Endoplasmic Reticulum Stress, Autophagy, and Neurodegeneration in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 691881. [Google Scholar] [CrossRef]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell Death Induced by Endoplasmic Reticulum Stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- Perier, C.; Bové, J.; Vila, M. Mitochondria and Programmed Cell Death in Parkinson’s Disease: Apoptosis and Beyond. Antioxidants Redox Signal. 2012, 16, 883–895. [Google Scholar] [CrossRef]
- Cerri, S.; Blandini, F. Role of Autophagy in Parkinson’s Disease. Curr. Med. Chem. 2019, 26, 3702–3718. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.; Fang, L. AMPK-SIRT1 Pathway Dysfunction Contributes to Neuron Apoptosis and Cognitive Impairment Induced by Sevoflurane. Mol. Med. Rep. 2020, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.J.; Codogno, P. AMP-Activated Protein Kinase and Autophagy. Autophagy 2007, 3, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Curry, B.; Mican, L.; Smith, T.L. Phenytoin-Induced Chronic Liver Enzyme Elevation and Hepatic Fibrosis: A Case Report. Ment. Health Clin. 2018, 8, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.B. Manganese Exposure, Essentiality & Toxicity. Indian J. Med. Res. 2008, 128, 484–500. [Google Scholar] [PubMed]
- Perl, D.P.; Olanow, C.W. The Neuropathology of Manganese-Induced Parkinsonism. J. Neuropathol. Exp. Neurol. 2007, 66, 675–682. [Google Scholar] [CrossRef]
- Nadeem, R.I.; Ahmed, H.I.; El-Sayeh, B.M. Protective Effect of Vinpocetine against Neurotoxicity of Manganese in Adult Male Rats. Naunyn Schmiedeberg’s Arch. Pharmacol. 2018, 391, 729–742. [Google Scholar] [CrossRef]
- Santini, Z.I.; Fiori, K.L.; Feeney, J.; Tyrovolas, S.; Haro, J.M.; Koyanagi, A. Social Relationships, Loneliness, and Mental Health among Older Men and Women in Ireland: A Prospective Community-Based Study. J. Affect. Disord. 2016, 204, 59–69. [Google Scholar] [CrossRef]
- Goh, Y.L.D.; Wilkinson, R.B. Attachment Strength and Relationship Expectancies in the Prediction of Adolescent Stress and Depression. Educ. Dev. Psychol. 2017, 34, 106–123. [Google Scholar] [CrossRef]
- Karkhanis, A.N.; Leach, A.C.; Yorgason, J.T.; Uneri, A.; Barth, S.; Niere, F.; Alexander, N.J.; Weiner, J.L.; McCool, B.A.; Raab-Graham, K.F.; et al. Chronic Social Isolation Stress during Peri-Adolescence Alters Presynaptic Dopamine Terminal Dynamics via Augmentation in Accumbal Dopamine Availability. ACS Chem. Neurosci. 2019, 10, 2033–2044. [Google Scholar] [CrossRef]
- Liu, J.; Dietz, K.; DeLoyht, J.M.; Pedre, X.; Kelkar, D.; Kaur, J.; Vialou, V.; Lobo, M.K.; Dietz, D.M.; Nestler, E.J.; et al. Impaired Adult Myelination in the Prefrontal Cortex of Socially Isolated Mice. Nat. Neurosci. 2012, 15, 1621–1623. [Google Scholar] [CrossRef]
- Matsuoka, H.; Yamaguchi, H. Path Dependence in Social and Psychological Risk Factors for Dementia. Dement. Neuropsychol. 2011, 5, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Cassano, T.; Calcagnini, S.; Carbone, A.; Bukke, V.N.; Orkisz, S.; Villani, R.; Romano, A.; Avolio, C.; Gaetani, S. Pharmacological Treatment of Depression in Alzheimer’s Disease: A Challenging Task. Front. Pharmacol. 2019, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Shulman, J.M.; De Jager, P.L.; Feany, M.B. Parkinson’s Disease: Genetics and Pathogenesis. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Shahpiri, Z.; Bahramsoltani, R.; Hosein Farzaei, M.; Farzaei, F.; Rahimi, R. Phytochemicals as Future Drugs for Parkinson’s Disease: A Comprehensive Review. Rev. Neurosci. 2016, 27, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Javanmard, S.; Zarfeshany, A. Potent Health Effects of Pomegranate. Adv. Biomed. Res. 2014, 3, 100. [Google Scholar] [CrossRef]
- Kim, Y.E.; Hwang, C.J.; Lee, H.P.; Kim, C.S.; Son, D.J.; Ham, Y.W.; Hellström, M.; Han, S.-B.; Kim, H.S.; Park, E.K.; et al. Inhibitory Effect of Punicalagin on Lipopolysaccharide-Induced Neuroinflammation, Oxidative Stress and Memory Impairment via Inhibition of Nuclear Factor-KappaB. Neuropharmacology 2017, 117, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Yaidikar, L.; Byna, B.; Thakur, S.R. Neuroprotective Effect of Punicalagin against Cerebral Ischemia Reperfusion-Induced Oxidative Brain Injury in Rats. J. Stroke Cerebrovasc. Dis. 2014, 23, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.-J.; Fischer, N.; Efferth, T. Phytochemicals as Inhibitors of NF-ΚB for Treatment of Alzheimer’s Disease. Pharmacol. Res. 2018, 129, 262–273. [Google Scholar] [CrossRef]
- Freeman, D.M.; O’Neal, R.; Zhang, Q.; Bouwer, E.J.; Wang, Z. Manganese-Induced Parkinsonism in Mice Is Reduced Using a Novel Contaminated Water Sediment Exposure Model. Environ. Toxicol. Pharmacol. 2020, 78, 103399. [Google Scholar] [CrossRef]
- Aboutaleb, A.; Ahmed, H.; Kamal, M.; Ali, A. Low Protein Diet: Its Relevance to Manganese-Induced Neurotoxicity in Rats Treated with Coenzyme Q10 and/or Epigallocatechin-3-Gallate. Azhar Int. J. Pharm. Med. Sci. 2021, 1, 32–55. [Google Scholar] [CrossRef]
- Fan, X.-M.; Luo, Y.; Cao, Y.-M.; Xiong, T.-W.; Song, S.; Liu, J.; Fan, Q.-Y. Chronic Manganese Administration with Longer Intervals Between Injections Produced Neurotoxicity and Hepatotoxicity in Rats. Neurochem. Res. 2020, 45, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, P.; Cordero, H.; Ijomone, O.M.; Ayodele, A.; Bornhorst, J.; Gunther, L.; Macaluso, F.P.; Bowman, A.B.; Aschner, M. Defective Mitochondrial Dynamics Underlie Manganese-Induced Neurotoxicity. Mol. Neurobiol. 2021, 58, 3270–3289. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.S.; Larsen, E.H.; Ladefoged, O.; Lam, H.R. Subchronic, Low-Level Intraperitoneal Injections of Manganese (IV) Oxide and Manganese (II) Chloride Affect Rat Brain Neurochemistry. Int. J. Toxicol. 2017, 36, 239–251. [Google Scholar] [CrossRef]
- Ali, A.A.; Ahmed, H.I.; Khaleel, S.A.; Abu-Elfotuh, K. Vinpocetine Mitigates Aluminum-Induced Cognitive Impairment in Socially Isolated Rats. Physiol. Behav. 2019, 208, 112571. [Google Scholar] [CrossRef]
- Kalshetti, P.; Alluri, R.; Mohan, V.; Thakurdesai, P. Effects of 4-Hydroxyisoleucine from Fenugreek Seeds on Depression-like Behavior in Socially Isolated Olfactory Bulbectomized Rats. Pharmacogn. Mag. 2015, 11, 388. [Google Scholar] [CrossRef]
- Bouabid, S.; Tinakoua, A.; Lakhdar-Ghazal, N.; Benazzouz, A. Manganese Neurotoxicity: Behavioral Disorders Associated with Dysfunctions in the Basal Ganglia and Neurochemical Transmission. J. Neurochem. 2016, 136, 677–691. [Google Scholar] [CrossRef]
- Peres, T.V.; Eyng, H.; Lopes, S.C.; Colle, D.; Gonçalves, F.M.; Venske, D.K.R.; Lopes, M.W.; Ben, J.; Bornhorst, J.; Schwerdtle, T.; et al. Developmental Exposure to Manganese Induces Lasting Motor and Cognitive Impairment in Rats. Neurotoxicology 2015, 50, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elfotuh, K.; Hamdan, A.M.E.; Abbas, A.N.; Alahmre, A.T.S.; Elewa, M.A.F.; Masoud, R.A.E.; Ali, A.A.; Othman, M.; Kamal, M.M.; Hassan, F.A.M.; et al. Evaluating the Neuroprotective Activities of Vinpocetine, Punicalagin, Niacin and Vitamin E against Behavioural and Motor Disabilities of Manganese-Induced Parkinson’s Disease in Sprague Dawley Rats. Biomed. Pharmacother. 2022, 153, 113330. [Google Scholar] [CrossRef] [PubMed]
- Yaidikar, L.; Thakur, S. Punicalagin Attenuated Cerebral Ischemia–Reperfusion Insult via Inhibition of Proinflammatory Cytokines, up-Regulation of Bcl-2, down-Regulation of Bax, and Caspase-3. Mol. Cell. Biochem. 2015, 402, 141–148. [Google Scholar] [CrossRef]
- Gao, Y.; Zhuang, Z.; Lu, Y.; Tao, T.; Zhou, Y.; Liu, G.; Wang, H.; Zhang, D.; Wu, L.; Dai, H.; et al. Curcumin Mitigates Neuro-Inflammation by Modulating Microglia Polarization Through Inhibiting TLR4 Axis Signaling Pathway Following Experimental Subarachnoid Hemorrhage. Front. Neurosci. 2019, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Hilakivi, L.A.; Lister, R.G. Correlations between Behavior of Mice in Porsolt’s Swim Test and in Tests of Anxiety, Locomotion, and Exploration. Behav. Neural Biol. 1990, 53, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.H.F.; Ahmed, A.-S.F.; Abdelrehim, A.B.; Heeba, G.H. The Impact of Single and Combined PPAR-α and PPAR-γ Activation on the Neurological Outcomes Following Cerebral Ischemia Reperfusion. Life Sci. 2020, 252, 117679. [Google Scholar] [CrossRef] [PubMed]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The Forced Swim Test as a Model of Depressive-like Behavior. J. Vis. Exp. 2015, 97, e52587. [Google Scholar] [CrossRef]
- Peng, W.-H.; Lo, K.-L.; Lee, Y.-H.; Hung, T.-H.; Lin, Y.-C. Berberine Produces Antidepressant-like Effects in the Forced Swim Test and in the Tail Suspension Test in Mice. Life Sci. 2007, 81, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; de Kloet, E.R. Immobility in the Forced Swim Test Is Adaptive and Does Not Reflect Depression. Psychoneuroendocrinology 2015, 62, 389–391. [Google Scholar] [CrossRef]
- Castagné, V.; Porsolt, R.D.; Moser, P. Use of Latency to Immobility Improves Detection of Antidepressant-like Activity in the Behavioral Despair Test in the Mouse. Eur. J. Pharmacol. 2009, 616, 128–133. [Google Scholar] [CrossRef]
- Hritcu, L.; Cioanca, O.; Hancianu, M. Effects of Lavender Oil Inhalation on Improving Scopolamine-Induced Spatial Memory Impairment in Laboratory Rats. Phytomedicine 2012, 19, 529–534. [Google Scholar] [CrossRef]
- Cooper, J.M.; Lathuiliere, A.; Migliorini, M.; Arai, A.L.; Wani, M.M.; Dujardin, S.; Muratoglu, S.C.; Hyman, B.T.; Strickland, D.K. Regulation of Tau Internalization, Degradation, and Seeding by LRP1 Reveals Multiple Pathways for Tau Catabolism. J. Biol. Chem. 2021, 296, 100715. [Google Scholar] [CrossRef]
- Michaelson, D.M. APOEε4: The Most Prevalent yet Understudied Risk Factor for Alzheimer’s Disease. Alzheimer’s Dement. 2014, 10, 861–868. [Google Scholar] [CrossRef]
- Pang, X.; Makinde, E.A.; Eze, F.N.; Olatunji, O.J. Securidaca Inappendiculata Polyphenol Rich Extract Counteracts Cognitive Deficits, Neuropathy, Neuroinflammation and Oxidative Stress in Diabetic Encephalopathic Rats via P38 MAPK/Nrf2/HO-1 Pathways. Front. Pharmacol. 2021, 12, 737764. [Google Scholar] [CrossRef]
- Ciarlone, A.E. Further Modification of a Fluorometric Method for Analyzing Brain Amines. Microchem. J. 1978, 23, 9–12. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. The Hematoxylins and Eosin. In Bancroft’s Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2013; pp. 173–186. [Google Scholar]
- Bancroft, J.; Gamble, M.A. Theory and Practice of Histological Techniques, 6th ed.; Elsevier Health Sciences, Ed.; Churchill Livingstone: London, UK, 2007. [Google Scholar]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current Understanding of the Molecular Mechanisms in Parkinson’s Disease: Targets for Potential Treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef]
- Raman, S.; Asle-Rousta, M.; Rahnema, M. Protective Effect of Fennel, and Its Major Component Trans-Anethole against Social Isolation Induced Behavioral Deficits in Rats. Physiol. Int. 2020, 107, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Jodynis-Liebert, J. Polyphenols in Parkinson’s Disease: A Systematic Review of In Vivo Studies. Nutrients 2018, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elfotuh, K.; Hamdan, A.M.E.; Mohammed, A.A.; Atwa, A.M.; Kozman, M.R.; Ibrahim, A.M.; Motawea, S.M.; Selim, H.M.R.M.; Tohamy, S.T.K.; Nour El-Din, M.N.; et al. Neuroprotective Effects of Some Nutraceuticals against Manganese-Induced Parkinson’s Disease in Rats: Possible Modulatory Effects on TLR4/NLRP3/NF-ΚB, GSK-3β, Nrf2/HO-1, and Apoptotic Pathways. Pharmaceuticals 2022, 15, 1554. [Google Scholar] [CrossRef]
- Karimian, M.; Famitafreshi, H.; Fanaei, H.; Attari, F.; Fatima, S. Social Isolation Is Associated with Reduced Neurogenesis, Impaired Spatial Working Memory Performance, and Altered Anxiety Levels in Male Rats. Open Access Anim. Physiol. 2015, 7, 87–95. [Google Scholar] [CrossRef]
- Bendersky, C.J.; Milian, A.A.; Andrus, M.D.; De La Torre, U.; Walker, D.M. Long-Term Impacts of Post-Weaning Social Isolation on Nucleus Accumbens Function. Front. Psychiatry 2021, 12, 745406. [Google Scholar] [CrossRef]
- Yan, M.H.; Wang, X.; Zhu, X. Mitochondrial Defects and Oxidative Stress in Alzheimer Disease and Parkinson Disease. Free Radic. Biol. Med. 2013, 62, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, S.; Chen, J.; Hu, K.; Li, Y.; Zhang, Z.; Su, Z.; Woodgett, J.R.; Li, M.; Huang, Q. GSK-3β Contributes to Parkinsonian Dopaminergic Neuron Death: Evidence From Conditional Knockout Mice and Tideglusib. Front. Mol. Neurosci. 2020, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Springer, K.; Gibson, J.S. Social Withdrawal in Parkinson’s Disease: A Scoping Review. Geriatr. Nurs. 2022, 48, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.; García-Gorostiaga, I.; Sánchez-Juan, P.; Sierra, M.; Martín-Gurpegui, J.L.; Terrazas, J.; Mateo, I.; Rodríguez-Rodríguez, E.; Berciano, J.; Combarros, O. Synergistic Effect of Two Oxidative Stress-Related Genes (Heme Oxygenase-1 and GSK3β) on the Risk of Parkinson’s Disease. Eur. J. Neurol. 2010, 17, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Tian, H.; Li, W.; Hung, H.; Sun, F. Potential Role of Punicalagin against Oxidative Stress Induced Testicular Damage. Asian J. Androl. 2016, 18, 627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, Q. Punicalagin Suppresses Inflammation in Ventilator-induced Lung Injury through Protease-activated Receptor-2 Inhibition-induced Inhibition of NLR Family Pyrin Domain Containing-3 Inflammasome Activation. Chem. Biol. Drug Des. 2022, 100, 218–229. [Google Scholar] [CrossRef]
- Gao, H.-M.; Zhou, H.; Zhang, F.; Wilson, B.C.; Kam, W.; Hong, J.-S. HMGB1 Acts on Microglia Mac1 to Mediate Chronic Neuroinflammation That Drives Progressive Neurodegeneration. J. Neurosci. 2011, 31, 1081–1092. [Google Scholar] [CrossRef]
- Azar, Y.O.; Badawi, G.A.; Zaki, H.F.; Ibrahim, S.M. Agmatine-Mediated Inhibition of NMDA Receptor Expression and Amelioration of Dyskinesia via Activation of Nrf2 and Suppression of HMGB1/RAGE/TLR4/MYD88/NF-ΚB Signaling Cascade in Rotenone Lesioned Rats. Life Sci. 2022, 311, 121049. [Google Scholar] [CrossRef]
- Ferrero-Andrés, A.; Panisello-Roselló, A.; Roselló-Catafau, J.; Folch-Puy, E. NLRP3 Inflammasome-Mediated Inflammation in Acute Pancreatitis. Int. J. Mol. Sci. 2020, 21, 5386. [Google Scholar] [CrossRef]
- Zádor, F.; Joca, S.; Nagy-Grócz, G.; Dvorácskó, S.; Szűcs, E.; Tömböly, C.; Benyhe, S.; Vécsei, L. Pro-Inflammatory Cytokines: Potential Links between the Endocannabinoid System and the Kynurenine Pathway in Depression. Int. J. Mol. Sci. 2021, 22, 5903. [Google Scholar] [CrossRef]
- Dehay, B.; Bourdenx, M.; Gorry, P.; Przedborski, S.; Vila, M.; Hunot, S.; Singleton, A.; Olanow, C.W.; Merchant, K.M.; Bezard, E.; et al. Targeting α-Synuclein for Treatment of Parkinson’s Disease: Mechanistic and Therapeutic Considerations. Lancet Neurol. 2015, 14, 855–866. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Y.; Yin, S.; Wan, F.; Hu, J.; Kou, L.; Sun, Y.; Wu, J.; Zhou, Q.; Huang, J.; et al. Targeting Microglial α-Synuclein/TLRs/NF-KappaB/NLRP3 Inflammasome Axis in Parkinson’s Disease. Front. Immunol. 2021, 12, 719807. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-J.; Yang, X.; Cui, H.-J.; Tang, T.; Zhong, J.-H.; Luo, J.-K.; Yang, A.-L.; Zhang, Q.-M.; Zhou, J.-H.; Zhang, Q. Leukemia Inhibitory Factor Contributes to Reactive Astrogliosis via Activation of Signal Transducer and Activator of Transcription 3 Signaling after Intracerebral Hemorrhage in Rats. J. Neurotrauma 2017, 34, 1658–1665. [Google Scholar] [CrossRef]
- Bousoik, E.; Montazeri Aliabadi, H. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front. Oncol. 2018, 8, 287. [Google Scholar] [CrossRef]
- Baker, B.J.; Akhtar, L.N.; Benveniste, E.N. SOCS1 and SOCS3 in the Control of CNS Immunity. Trends Immunol. 2009, 30, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, H.; He, Y.; Fu, S.; Liu, H.; Wang, Q.; Shen, J. Proteomics-Guided Study on Buyang Huanwu Decoction for Its Neuroprotective and Neurogenic Mechanisms for Transient Ischemic Stroke: Involvements of EGFR/PI3K/Akt/Bad/14-3-3 and Jak2/Stat3/Cyclin D1 Signaling Cascades. Mol. Neurobiol. 2020, 57, 4305–4321. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yang, Q.; Wang, C.; Tong, W.; Xu, W. Punicalagin Exerts Protective Effects against Ankylosing Spondylitis by Regulating NF- κ B-TH17/JAK2/STAT3 Signaling and Oxidative Stress. BioMed Res. Int. 2020, 2020, 4918239. [Google Scholar] [CrossRef] [PubMed]
- Hendawy, N.; Salaheldin, T.H.; Abuelezz, S.A. PCSK9 Inhibition Reduces Depressive like Behavior in CUMS-Exposed Rats: Highlights on HMGB1/RAGE/TLR4 Pathway, NLRP3 Inflammasome Complex and IDO-1. J. Neuroimmune Pharmacol. 2023, 18, 195–207. [Google Scholar] [CrossRef]
- Han, Y.; Chen, R.; Lin, Q.; Liu, Y.; Ge, W.; Cao, H.; Li, J. Curcumin Improves Memory Deficits by Inhibiting HMGB1-RAGE/TLR4-NF-κB Signalling Pathway in APPswe/PS1dE9 Transgenic Mice Hippocampus. J. Cell. Mol. Med. 2021, 25, 8947–8956. [Google Scholar] [CrossRef]
- Bellezza, I.; Tucci, A.; Galli, F.; Grottelli, S.; Mierla, A.L.; Pilolli, F.; Minelli, A. Inhibition of NF-ΚB Nuclear Translocation via HO-1 Activation Underlies α-Tocopheryl Succinate Toxicity. J. Nutr. Biochem. 2012, 23, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Zhang, Y.; Cao, Y.; Chen, J.; Qin, H.; Yang, L. Punicalagin Protects Diabetic Nephropathy by Inhibiting Pyroptosis Based on TXNIP/NLRP3 Pathway. Nutrients 2020, 12, 1516. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, K.; Wu, X.; Xin, C.; Zhou, M.; Lei, J.; Chen, J. Punicalagin Alleviates Brain Injury and Inflammatory Responses, and Regulates HO-1/Nrf-2/ARE Signaling in Rats after Experimental Intracerebral Haemorrhage. Trop. J. Pharm. Res. 2020, 19, 727–737. [Google Scholar] [CrossRef]
- Makled, M.N.; El-Awady, M.S.; Abdelaziz, R.R.; Atwan, N.; Guns, E.T.; Gameil, N.M.; Shehab El-Din, A.B.; Ammar, E.M. Pomegranate Protects Liver against Cecal Ligation and Puncture-Induced Oxidative Stress and Inflammation in Rats through TLR 4 /NF-κB Pathway Inhibition. Environ. Toxicol. Pharmacol. 2016, 43, 182–192. [Google Scholar] [CrossRef]
- Hang, W.; Fan, H.; Li, Y.; Xiao, Q.; Jia, L.; Song, L.; Gao, Y.; Jin, X.; Xiao, B.; Yu, J.; et al. Wuzi Yanzong Pill Attenuates MPTP-Induced Parkinson’s Disease via PI3K/Akt Signaling Pathway. Metab. Brain Dis. 2022, 37, 1435–1450. [Google Scholar] [CrossRef]
- Rekha, K.R.; Selvakumar, G.P. Gene Expression Regulation of Bcl2, Bax and Cytochrome-C by Geraniol on Chronic MPTP/Probenecid Induced C57BL/6 Mice Model of Parkinson’s Disease. Chem. Biol. Interact. 2014, 217, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-Derived Neurotrophic Factor and Its Clinical Implications. Arch. Med. Sci. 2015, 6, 1164–1178. [Google Scholar] [CrossRef]
- Li, D.-W.; Liu, Z.-Q.; Chen, W.; Yao, M.; Li, G.-R. Association of Glycogen Synthase Kinase-3β with Parkinson’s Disease (Review). Mol. Med. Rep. 2014, 9, 2043–2050. [Google Scholar] [CrossRef]
- Noori, T.; Dehpour, A.R.; Sureda, A.; Sobarzo-Sanchez, E.; Shirooie, S. Role of Natural Products for the Treatment of Alzheimer’s Disease. Eur. J. Pharmacol. 2021, 898, 173974. [Google Scholar] [CrossRef]
- Gouda, N.A.; Cho, J. Omarigliptin Mitigates 6-Hydroxydopamine- or Rotenone-Induced Oxidative Toxicity in PC12 Cells by Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Actions. Antioxidants 2022, 11, 1940. [Google Scholar] [CrossRef]
- Soni, D.; Kumar, P. GSK-3β-Mediated Regulation of Nrf2/HO-1 Signaling as a New Therapeutic Approach in the Treatment of Movement Disorders. Pharmacol. Rep. 2022, 74, 557–569. [Google Scholar] [CrossRef]
- Adams, C.J.; Kopp, M.C.; Larburu, N.; Nowak, P.R.; Ali, M.M.U. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front. Mol. Biosci. 2019, 6, 11. [Google Scholar] [CrossRef]
- Fouillet, A.; Levet, C.; Virgone, A.; Robin, M.; Dourlen, P.; Rieusset, J.; Belaidi, E.; Ovize, M.; Touret, M.; Nataf, S.; et al. ER Stress Inhibits Neuronal Death by Promoting Autophagy. Autophagy 2012, 8, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Lindholm, D.; Ren, J.; Pratico, D. ER Stress and UPR in Alzheimer’s Disease: Mechanisms, Pathogenesis, Treatments. Cell Death Dis. 2022, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, A.R.; Giordano, F.; Gerlo, S.; Segura, I.; Van Eygen, S.; Molenberghs, G.; Rocha, S.; Houcine, A.; Derua, R.; Verfaillie, T.; et al. The ER Stress Sensor PERK Coordinates ER-Plasma Membrane Contact Site Formation through Interaction with Filamin-A and F-Actin Remodeling. Mol. Cell 2017, 65, 885–899.e6. [Google Scholar] [CrossRef] [PubMed]
- Romine, I.C.; Wiseman, R.L. PERK Signaling Regulates Extracellular Proteostasis of an Amyloidogenic Protein During Endoplasmic Reticulum Stress. Sci. Rep. 2019, 9, 410. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Ying, C.; Li, W.; Ruan, H.; Zhu, X.; You, Y.; Han, Y.; Chen, R.; Wang, Y.; et al. Inhibition of Glycogen Synthase Kinase-3β Protects Dopaminergic Neurons from MPTP Toxicity. Neuropharmacology 2007, 52, 1678–1684. [Google Scholar] [CrossRef]
- Zhong, J.; Reece, E.A.; Yang, P. Punicalagin Exerts Protective Effect against High Glucose-Induced Cellular Stress and Neural Tube Defects. Biochem. Biophys. Res. Commun. 2015, 467, 179–184. [Google Scholar] [CrossRef]
- Mo, F.; Lv, B.; An, T.; Miao, J.; Liu, J.; Zhang, J.; Zhang, Z.; Ma, M.; Yang, X.; Zhao, D.; et al. Protective Mechanism of Punicalagin against Endoplasmic Reticulum Stress in the Liver of Mice with Type 2 Diabetes Mellitus. J. Funct. Foods 2019, 56, 57–64. [Google Scholar] [CrossRef]
- El-Missiry, M.A.; ElKomy, M.A.; Othman, A.I.; AbouEl-ezz, A.M. Punicalagin Ameliorates the Elevation of Plasma Homocysteine, Amyloid-β, TNF-α and Apoptosis by Advocating Antioxidants and Modulating Apoptotic Mediator Proteins in Brain. Biomed. Pharmacother. 2018, 102, 472–480. [Google Scholar] [CrossRef]
- Santos, R.X.; Correia, S.C.; Carvalho, C.; Cardoso, S.; Santos, M.S.; Moreira, P.I. Mitophagy in Neurodegeneration: An Opportunity for Therapy? Curr. Drug Targets 2011, 12, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Fonseca, T.; Villar-Piqué, A.; Outeiro, T. The Interplay between Alpha-Synuclein Clearance and Spreading. Biomolecules 2015, 5, 435–471. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK Signalling Pathway Coordinates Cell Growth, Autophagy and Metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Parekh, P.; Sharma, N.; Gadepalli, A.; Shahane, A.; Sharma, M.; Khairnar, A. A Cleaning Crew: The Pursuit of Autophagy in Parkinson’s Disease. ACS Chem. Neurosci. 2019, 10, 3914–3926. [Google Scholar] [CrossRef] [PubMed]
- Hang, L.; Wang, Z.; Foo, A.S.C.; Goh, G.W.Y.; Choong, H.C.; Thundyil, J.; Xu, S.; Lam, K.-P.; Lim, K.-L. Conditional Disruption of AMP Kinase in Dopaminergic Neurons Promotes Parkinson’s Disease-Associated Phenotypes in Vivo. Neurobiol. Dis. 2021, 161, 105560. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Wang, Y.; Sun, D.; Liu, Z.; Wang, J.; Li, X.; Huo, C.; Jia, X.; Chen, W.; Fu, F.; et al. Punicalagin Pretreatment Attenuates Myocardial Ischemia-Reperfusion Injury via Activation of AMPK. Am. J. Chin. Med. 2017, 45, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, H.; Yin, S.; Hu, H.; Zhao, C. Punicalagin Inhibits Palmitate Acid-Induced Lipoapoptosis through Inhibition of ER Stress, and Activation of SIRT1/Autophagy in HepG2 Cells. J. Food Nutr. Res. 2020, 8, 536–542. [Google Scholar] [CrossRef]
- Yu, L.-M.; Dong, X.; Xue, X.-D.; Zhang, J.; Li, Z.; Wu, H.-J.; Yang, Z.-L.; Yang, Y.; Wang, H.-S. Protection of the Myocardium against Ischemia/Reperfusion Injury by Punicalagin through an SIRT1-NRF-2-HO-1-Dependent Mechanism. Chem. Biol. Interact. 2019, 306, 152–162. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, Y.; Wan, F.; Ma, K.; Guo, X.; Kou, L.; Yin, S.; Han, C.; Liu, L.; Huang, J.; et al. New Perspectives on Roles of Alpha-Synuclein in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 370. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Zhu, Y.; Li, Z.; Zhu, Y.; Wu, J.; Qin, Z.; Xiang, M.; Lin, F. Exercise Activates Lysosomal Function in the Brain through AMPK-SIRT1-TFEB Pathway. CNS Neurosci. Ther. 2019, 25, 796–807. [Google Scholar] [CrossRef]
- Phillipson, O.T. Alpha-Synuclein, Epigenetics, Mitochondria, Metabolism, Calcium Traffic, & Circadian Dysfunction in Parkinson’s Disease. An Integrated Strategy for Management. Ageing Res. Rev. 2017, 40, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Fatah, I.M.; Abdelrazek, H.M.A.; Ibrahim, S.M.; Abdallah, D.M.; El-Abhar, H.S. Dimethyl Fumarate Abridged Tauo-/Amyloidopathy in a D-Galactose/Ovariectomy-Induced Alzheimer’s-like Disease: Modulation of AMPK/SIRT-1, AKT/CREB/BDNF, AKT/GSK-3β, Adiponectin/Adipo1R, and NF-ΚB/IL-1β/ROS Trajectories. Neurochem. Int. 2021, 148, 105082. [Google Scholar] [CrossRef] [PubMed]










| Target Gene | Gene Forward and Backward Primer Sequence |
|---|---|
| Bax | F: 5′-ATGTTTTCTGACGGCAACTTC-3′ R: 5′-AGTCCAATGTCCAGCCCAT-3′ |
| Bcl-2 | F: 5′-CTACGAGTGGGATGCTGGAG-3′ R: 5′-TTCTTCACGATGGTGAGCG-3′ |
| Caspase-3 | F: 5′-GTGGAACTGACGATGATATGGC-3′ R: 5′-CGCAAAGTGACTGGATGAACC-3′ |
| Caspase-1 | F:5′-GAACAAAGAAGGTGGCGCAT-3′ R:5′-GAGGTCAACATCAGCTCCGA-3′ |
| NLRP3 | F:5′-TGCATGCCGTATCTGGTTGT-3′ R:5′-ACCTCTTGCGAGGGTCTTTG-3′ |
| NF-Κβ | F:5′-CGCGGGGACTATGACTTGAA-3′ R:5′-AGTTCCGGTTTACTCGGCAG-3′ |
| HO-1 | F:5′-CACCAGCCACACAGCACTAC-3′ R: 5′-CACCCACCCCTCAAAAGACA-3′ |
| Nrf2 | F:5′-CTCTCTGGAGACGGCCATGACT-3′ R:5′-CTGGGCTGGGGACAGTGGTAGT-3′ |
| TLR4 | F: 5′-TCAGCTTTGGTCAGTTGGCT-3′ R: 5′-GTCCTTGACCCACTGCAAGA-3′ |
| GSK-3β | F: 5′-ACACACCTGCCCTCTTCAAC-3′ R: 5′-GAAGCGGCGTTATTGGTCTG-3′ |
| PERK | F: 5′-GCCGATGGGATAGTGATG-3′ R: 5′-GCAGCCTCTACAATGTCTTCT-3′ |
| CHOP | F: 5′-TCTGCCTTTCGCCTTTGAG-3′ R: 5′-GCTTTGGGAGGTGCTTGTG-3′ |
| GRP78 | F:5′-GACATCAAGTTCTTGCCGTT-3′ R:5′-CTCATAACATTTAGGCCAGC-3′ |
| PI3K | F: 5′-GCCCAGGCTTACTACAGAC-3′ R: 5′-AAGTAGGGAGGCATCTCG-3′ |
| TrkB | F: 5′- CCTCCACGGATGTTGCTGA-3′ R: 5′-GGCTGTTGGTGATACCGAAGTA-3′ |
| AMPK | F: 5′ -AAAGAACCCTAGCCTGAAGAGG-3′ R: 5′-ACCTTCCGAGATGAATGCTTTT-3′ |
| SIRT 1 | F: 5′- GGCACCGATCCTCGAACAAT-3′ R: 5′-CGCTTTGGTGGTTCTGAAAGG-3′ |
| Beclin-1 | F: 5′-AGCACGCCATGTATAGCAAAGA-3′ R:5′-GGAAGAGGGAAAGGACAGCAT-3′ |
| AKT | F: 5′-AGGAGGAGGAGGAGATGGA-3′ R: 5′-GGTCGTGGGTCTGGAAAG-3′ |
| CREB | F: 5′-CAGACAACCAGCAGAGTGGA-3′ R: 5′-CTGGACTGTCTGCCCATTG-3′ |
| HMGB1 | F: 5′-CACCCTGCATATTGTGGTAGG-3′ R: 5′-CGCTGGGACTAAGGTCAACA-3′ |
| RAGE | F: 5′-GAGTCCGAGTCTACCAGATTCC-3′ R:5′-GGTCTCCTCCTTCACAACTGTC-3′ |
| JAK-2 | F: 5′-AGCTCCTCTCCTTGACGACT-3′ R:5′-GCACGCACTTCGGTAAGAAC-3′ |
| STAT-3 | F: 5′-CAAAGAAAACATGGCCGGCA-3′ R:5′-GGGGGCTTTGTGCTTAGGAT-3′ |
| GAPDH | F:5′-AACTCCCATTCCTCCACCTT-3′ R:5-GAGGGCCTCTCTCTTGCTCT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, H.A.; Abu-Elfotuh, K.; Alzahrani, S.; Rizk, N.I.; Ali, H.S.; Elsherbiny, N.; Aljohani, A.; Hamdan, A.M.E.; Chellasamy, P.; Abdou, N.S.; et al. Punicalagin’s Protective Effects on Parkinson’s Progression in Socially Isolated and Socialized Rats: Insights into Multifaceted Pathway. Pharmaceutics 2023, 15, 2420. https://doi.org/10.3390/pharmaceutics15102420
Salem HA, Abu-Elfotuh K, Alzahrani S, Rizk NI, Ali HS, Elsherbiny N, Aljohani A, Hamdan AME, Chellasamy P, Abdou NS, et al. Punicalagin’s Protective Effects on Parkinson’s Progression in Socially Isolated and Socialized Rats: Insights into Multifaceted Pathway. Pharmaceutics. 2023; 15(10):2420. https://doi.org/10.3390/pharmaceutics15102420
Chicago/Turabian StyleSalem, Hoda A., Karema Abu-Elfotuh, Sharifa Alzahrani, Nermin I. Rizk, Howaida S. Ali, Nehal Elsherbiny, Alhanouf Aljohani, Ahmed M. E. Hamdan, Panneerselvam Chellasamy, Nada S. Abdou, and et al. 2023. "Punicalagin’s Protective Effects on Parkinson’s Progression in Socially Isolated and Socialized Rats: Insights into Multifaceted Pathway" Pharmaceutics 15, no. 10: 2420. https://doi.org/10.3390/pharmaceutics15102420
APA StyleSalem, H. A., Abu-Elfotuh, K., Alzahrani, S., Rizk, N. I., Ali, H. S., Elsherbiny, N., Aljohani, A., Hamdan, A. M. E., Chellasamy, P., Abdou, N. S., Gowifel, A. M. H., Darwish, A., Ibrahim, O. M., & Abd Elmageed, Z. Y. (2023). Punicalagin’s Protective Effects on Parkinson’s Progression in Socially Isolated and Socialized Rats: Insights into Multifaceted Pathway. Pharmaceutics, 15(10), 2420. https://doi.org/10.3390/pharmaceutics15102420