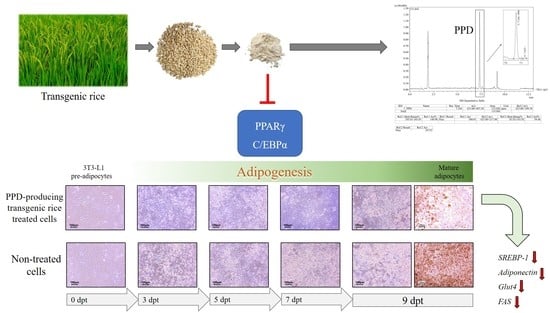
Protopanaxadiol-Enriched Rice Exerted Antiadipogenic Activity during 3T3-L1 Differentiation and Anti-Inflammatory Activity in 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Treatment Preparation
2.2. 3T3-L1 Cell Culture
2.3. Preadipocyte Differentiation
2.4. Cell Viability Assay
2.5. Oil Red O Assay
2.6. RNA Extraction and Real-Time PCR Analysis
2.7. Western Blotting Analysis
2.8. 3T3-L1 Cell Differentiation and LPS-Stimulated Adipocyte Viability Assay
2.9. Real-Time PCR and Western Blotting in LPS-Stimulated Adipocytes
2.10. Cell Cycle Assay
2.11. Statistical Analysis
3. Results
3.1. Effects of PPD-Containing Rice Seed Extracts on Cell Viability
3.2. Effects of PPD-Containing Rice Seed Extracts on Lipid Accumulation
3.3. Effects of PPD-Containing Rice Seed Extracts on Cell Differentiation
3.4. Effects of PPD-Containing Rice Seed Extracts on Adipogenic Gene Expression
3.5. Effects of PPD-Containing Rice Seed Extracts on Protein Expression
3.6. Effects of PPD-Containing Rice Seed Extracts on the Viability of LPS-Stimulated Adipocytes
3.7. Effects of PPD-Containing Rice Seed Extracts on Immune-Related mRNA in LPS-Stimulated Adipocytes
3.8. Effects of PPD-Containing Rice Seed Extracts on the Activation of Immune-Related Pathways in LPS-Stimulated Adipocytes
3.9. Effects of PPD-Containing Rice Seed Extracts on Cell Cycle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a potential anti-obesity target: A review of pharmacological treatment and natural products. Diabetes Metab. Syndr. Obes. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Kim, H.L.; Lee, S.K.; Min, D.E.; Choi, B.K.; Lee, D.R. Anti-obesity effects of a mixture of Atractylodes macrocephala and Amomum villosum extracts on 3T3-L1 adipocytes and high-fat diet-induced obesity in mice. Molecules 2022, 27, 906. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’i, A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021, 136, 104754–104770. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Liu, K.; Feng, J.; Zheng, Z.; Xie, X. Prevalence and risk factors of type 2 diabetes and prediabetes among 53,288 middle-aged and elderly adults in China: A cross-sectional study. Diabetes Metab. Syndr. Obes. 2021, 14, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, T.; Ochiai, H.; Yoshimoto, T.; Nagahama, S.; Kobayashi, M.; Ohtsu, I.; Sunaga, Y.; Kokaze, A. Associations between normal weight central obesity and cardiovascular disease risk factors in Japanese middle-aged adults: A cross-sectional study. J. Health Popul. Nutr. 2019, 38, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Parikesit, D.; Mochtar, C.A.; Umbas, R.; Hamid, A.R.A.H. The impact of obesity towards prostate diseases. Prostate Int. 2016, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Xanthopoulos, M.; Tapia, I.E. Obesity and common respiratory diseases in children. Paediatr. Respir. Rev. 2017, 23, 68–71. [Google Scholar] [CrossRef]
- Farmer, S.R. Regulation of PPARγ activity during adipogenesis. Int. J. Obes. 2005, 29, S13–S16. [Google Scholar] [CrossRef] [Green Version]
- Hadrich, F.; Sayadi, S. Apigetrin inhibits adipogenesis in 3T3-L1 cells by downregulating PPARγ and CEBP-α. Lipids Health Dis. 2018, 17, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.-H.; Jang, J.; Chung, S.-I.; Yoon, Y. AMPK and SREBP-1c mediate the anti-adipogenic effect of β-hydroxyisovalerylshikonin. Int. J. Mol. Med. 2016, 37, 816–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceja-Galicia, Z.; Calderón-DuPont, D.; Daniel, A.; Chiu, L.M.; Díaz-Villaseñor, A. Leptin and adiponectin synthesis and secretion in mature 3T3-L1 adipocytes are differentially down-regulated by arsenic and palmitic acid exposure throughout different stages of adipogenesis. Life Sci. 2022, 291, 120262–120275. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Cha, B.-Y.; Iida, K.; Lee, Y.-S.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.-T. Artepillin C, as a PPARγ ligand, enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells. Biochem. Pharmacol. 2011, 81, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.U.; Kim, H.J.; Kim, D.H.; Han, C.H.; Lee, Y.S.; Kim, C.-H. Nonthermal plasma treated solution inhibits adipocyte differentiation and lipogenesis in 3T3-L1 preadipocytes via ER stress signal suppression. Sci. Rep. 2018, 8, 2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siraj, F.M.; SathishKumar, N.; Kim, Y.J.; Kim, S.Y.; Yang, D.C. Ginsenoside F2 possesses anti-obesity activity via binding with PPARγ and inhibiting adipocyte differentiation in the 3T3-L1 cell line. J. Enzym. Inhib. Med. Chem. 2015, 30, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.Y.; Kim, A.R.; Yoo, E.S.; Baik, K.U.; Park, M.H. Ginsenosides from Panax ginseng differentially regulate lymphocyte proliferation. Planta Med. 2002, 68, 497–500. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, J.; Rhee, M.H.; Yu, T.; Baek, K.-S.; Sung, N.Y.; Kim, Y.; Yoon, K.; Kim, J.H.; Kwak, Y.-S.; et al. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J. Ginseng. Res. 2015, 39, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H. Cardiovascular diseases and Panax ginseng: A review on molecular mechanisms and medical applications. J. Ginseng. Res. 2012, 36, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Kim, J.-H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng. Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.N.; Moon, S.J.; Ko, S.K.; Im, B.O.; Chung, S.H. Wild ginseng prevents the onset of high-fat diet induced hyperglycemia and obesity in icr mice. Arch. Pharm. Res. 2004, 27, 790–796. [Google Scholar] [CrossRef]
- Kim, S.N.; Lee, J.H.; Shin, H.; Son, S.H.; Kim, Y.S. Effects of in vitro-digested ginsenosides on lipid accumulation in 3T3-L1 adipocytes. Planta Med. 2009, 75, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.J.; Oh, J.S.; Park, M.S.; Ji, G.E. Anti-hyperglycemic effect of fermented ginseng in type 2 diabetes mellitus mouse model. Phytother. Res. 2013, 27, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Aung, H.H.; Zhang, B.I.N.; Sun, S.H.I.; Li, X.-L.; He, H.U.I.; Xie, J.-T.; He, T.-C.; Du, W.E.I.; Yuan, C.-S. Chemopreventive effects of heat-processed Panax quinquefolius root on human breast cancer cells. Anticancer Res. 2008, 28, 2545–2551. [Google Scholar]
- Oh, H.A.; Kim, D.-E.; Choi, H.J.; Kim, N.J.; Kim, D.-H. Anti-stress effects of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol in immobilized Mice. Biol. Pharm. Bull. 2015, 38, 331–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-Y.; Cho, J.Y. 20S-dihydroprotopanaxadiol, a ginsenoside derivative, boosts innate immune responses of monocytes and macrophages. J. Ginseng. Res. 2013, 37, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.T.; Lee, M.S.; Kim, H.J.; Sung, M.J.; Kim, H.Y.; Kim, M.S.; Kwon, D.Y. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-gamma signal pathways. Phytother. Res. 2009, 23, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Baek, S.H.; Jo, H.J.; Yun, D.W.; Choi, Y.E. Genetically modified rice produces ginsenoside aglycone (protopanaxadiol). Planta 2019, 250, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Monmai, C.; Kim, J.-S.; Baek, S.-H. Transgenic rice seed extracts exert immunomodulatory effects by modulating immune-related biomarkers in RAW264.7 macrophage cells. Nutrients 2022, 14, 4143. [Google Scholar] [CrossRef]
- Monmai, C.; Kim, J.-S.; Baek, S.-H. Effect of ginseng sapogenin protopanaxadiol-enriched rice (DJ-PPD) on immunomodulation. Plants 2023, 12, 767. [Google Scholar] [CrossRef]
- Monmai, C.; Kim, J.-S.; Promyot, K.; Baek, S.-H. Protopanaxadiol-enriched rice extracts suppressed oxidative and melanogenic activities in melan-a cells. Antioxidants 2023, 12, 166. [Google Scholar] [CrossRef]
- Lee, W.; Song, G.; Bae, H. Suppressive effect of fraxetin on adipogenesis and reactive oxygen species production in 3T3-L1 cells by regulating MAPK signaling pathways. Antioxidants 2022, 11, 1893. [Google Scholar] [CrossRef] [PubMed]
- Gurriarán-Rodríguez, U.; Al-Massadi, O.; Roca-Rivada, A.; Crujeiras, A.B.; Gallego, R.; Pardo, M.; Seoane, L.M.; Pazos, Y.; Casanueva, F.F.; Camiña, J.P. Obestatin as a regulator of adipocyte metabolism and adipogenesis. J. Cell. Mol. Med. 2011, 15, 1927–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cignarelli, A.; Genchi, V.A.; Perrini, S.; Natalicchio, A.; Laviola, L.; Giorgino, F. Insulin and insulin receptors in adipose tissue development. Int. J. Mol. Sci. 2019, 20, 759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPalpha induces adipogenesis through PPARgamma: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, L.; Wang, X.; Si, H. Anti-adipogenic effects and mechanisms of ginsenoside Rg3 in pre-adipocytes and obese mice. Front. Pharmacol. 2017, 8, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.W.; Kim, S.S. Ginsenoside Rc promotes anti-adipogenic activity on 3T3-L1 adipocytes by down-regulating C/EBPα and PPARγ. Molecules 2015, 20, 1293–1303. [Google Scholar] [CrossRef] [Green Version]
- Aouadi, M.; Laurent, K.; Prot, M.; Le Marchand-Brustel, Y.; Binétruy, B.; Bost, F. Inhibition of p38MAPK increases adipogenesis from embryonic to adult stages. Diabetes 2006, 55, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Belmonte, N.; Phillips, B.W.; Massiera, F.; Villageois, P.; Wdziekonski, B.; Saint-Marc, P.; Nichols, J.; Aubert, J.; Saeki, K.; Yuo, A.; et al. Activation of extracellular signal-regulated kinases and CREB/ATF-1 mediate the expression of CCAAT/enhancer binding proteins beta and -delta in preadipocytes. Mol. Endocrinol. 2001, 15, 2037–2049. [Google Scholar] [CrossRef] [Green Version]
- Prusty, D.; Park, B.H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef] [Green Version]
- Donzelli, E.; Lucchini, C.; Ballarini, E.; Scuteri, A.; Carini, F.; Tredici, G.; Miloso, M. ERK1 and ERK2 are involved in recruitment and maturation of human mesenchymal stem cells induced to adipogenic differentiation. J. Mol. Cell Biol. 2011, 3, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.X.; Chen, G.; Yin, Z.Y.; Zhang, Y.H.; Zheng, X.X. p-Synephrine exhibits anti-adipogenic activity by activating the Akt/GSK3β signaling pathway in 3T3-L1 adipocytes. J. Food Biochem. 2019, 43, e13033. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, K.; Dong, H.; Yang, J.; Yoshizawa, M.; Kagami, H.; Li, X. Alliin inhibits adipocyte differentiation by downregulating Akt expression: Implications for metabolic disease. Exp. Ther. Med. 2021, 21, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wu, B.; Xu, W.; Jin, X.; Wang, K.; Wang, H. The inhibitory effects of Juglanin on adipogenesis in 3T3-L1 adipocytes. Drug Des. Devel. Ther. 2020, 14, 5349–5357. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Young-Cheul, K. Adipocyte differentiation and gene expression. J. Nutr. 2000, 130, 3122s–3126s. [Google Scholar] [CrossRef]
- Tomasello, B.; Malfa, G.A.; La Mantia, A.; Miceli, N.; Sferrazzo, G.; Taviano, M.F.; Di Giacomo, C.; Renis, M.; Acquaviva, R. Anti-adipogenic and anti-oxidant effects of a standardised extract of Moro blood oranges (Citrus sinensis (L.) Osbeck) during adipocyte differentiation of 3T3-L1 preadipocytes. Nat. Prod. Res. 2021, 35, 2660–2667. [Google Scholar] [CrossRef]
- Lee, J.B.; Yoon, S.J.; Lee, S.H.; Lee, M.S.; Jung, H.; Kim, T.D.; Yoon, S.R.; Choi, I.; Kim, I.S.; Chung, S.W.; et al. Ginsenoside Rg3 ameliorated HFD-induced hepatic steatosis through downregulation of STAT5-PPARγ. J. Endocrinol. 2017, 235, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Schäffler, A.; Müller-Ladner, U.; Schölmerich, J.; Büchler, C. Role of adipose tissue as an inflammatory organ in human diseases. Endocr. Rev. 2006, 27, 449–467. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.L.; Ha, A.W.; Kim, W.K. Effect of saccharin on inflammation in 3T3-L1 adipocytes and the related mechanism. Nutr. Res. Pract. 2020, 14, 109–116. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–920. [Google Scholar] [CrossRef]
- Cho, J.W.; Lee, K.S.; Kim, C.W. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int. J. Mol. Med. 2007, 19, 469–474. [Google Scholar]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Ballak, D.B.; Stienstra, R.; Tack, C.J.; Dinarello, C.A.; van Diepen, J.A. IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine 2015, 75, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.Y.; Kim, J.-H.; Jung, M.H. Anti-obesity effects of tanshinone I from Salvia miltiorrhiza Bunge in mice fed a high-fat diet through inhibition of early adipogenesis. Nutrients 2020, 12, 1242. [Google Scholar] [CrossRef] [PubMed]











| Treatment | PPD Content (µg/g Dry Weight of Extract) |
|---|---|
| DJ | n.d. |
| #8 | 7.28 ± 0.64 |
| #503 | 1.13 ± 0.03 |
| #577 | 1.30 ± 0.03 |
| #564 | 1.28 ± 0.08 |
| #595 | 2.36 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monmai, C.; Kim, J.-S.; Sim, H.B.; Yun, D.-W.; Oh, S.-D.; Rha, E.-S.; Kim, J.-J.; Baek, S.-H. Protopanaxadiol-Enriched Rice Exerted Antiadipogenic Activity during 3T3-L1 Differentiation and Anti-Inflammatory Activity in 3T3-L1 Adipocytes. Pharmaceutics 2023, 15, 2123. https://doi.org/10.3390/pharmaceutics15082123
Monmai C, Kim J-S, Sim HB, Yun D-W, Oh S-D, Rha E-S, Kim J-J, Baek S-H. Protopanaxadiol-Enriched Rice Exerted Antiadipogenic Activity during 3T3-L1 Differentiation and Anti-Inflammatory Activity in 3T3-L1 Adipocytes. Pharmaceutics. 2023; 15(8):2123. https://doi.org/10.3390/pharmaceutics15082123
Chicago/Turabian StyleMonmai, Chaiwat, Jin-Suk Kim, Hyun Bo Sim, Doh-Won Yun, Sung-Dug Oh, Eui-Shik Rha, Jong-Jin Kim, and So-Hyeon Baek. 2023. "Protopanaxadiol-Enriched Rice Exerted Antiadipogenic Activity during 3T3-L1 Differentiation and Anti-Inflammatory Activity in 3T3-L1 Adipocytes" Pharmaceutics 15, no. 8: 2123. https://doi.org/10.3390/pharmaceutics15082123
APA StyleMonmai, C., Kim, J. -S., Sim, H. B., Yun, D. -W., Oh, S. -D., Rha, E. -S., Kim, J. -J., & Baek, S. -H. (2023). Protopanaxadiol-Enriched Rice Exerted Antiadipogenic Activity during 3T3-L1 Differentiation and Anti-Inflammatory Activity in 3T3-L1 Adipocytes. Pharmaceutics, 15(8), 2123. https://doi.org/10.3390/pharmaceutics15082123