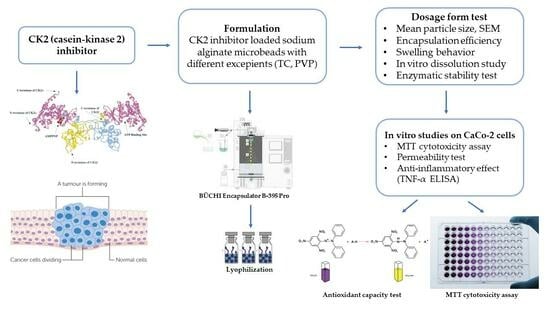
Formulation and Investigation of CK2 Inhibitor-Loaded Alginate Microbeads with Different Excipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation of CK2 Inhibitor-Loaded Alginate Beads
2.3. Mean Particle Size and SEM
2.4. Encapsulation Efficiency
2.5. Swelling Behavior
2.6. Enzymatic Stability Test
2.7. In Vitro Dissolution Study
2.8. MTT Assay
2.9. Transepithelial Electrical Resistance Measurement
2.10. In Vitro Permeability Studies
2.11. HPLC Measurements
2.12. DPPH Anti-Oxidant Test
2.13. Examination of Anti-Inflammatory Effect
2.14. Statistical Analysis
3. Results
3.1. In Silico Physicochemical Characterization of DMAT
3.2. Mean Particle Size and SEM
3.3. Encapsulation Efficiency
3.4. Swelling Behavior
3.5. Result of Enzymatic Stability Test
3.6. In Vitro Dissolution Study
3.7. MTT Assay
3.8. In Vitro Permeability Test
3.9. Transepithelial Electrical Resistance Measurements
3.10. DPPH Scavenging Activity Test
3.11. Examination of In Vitro Anti-Inflammatory Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Silva-Pavez, E.; Tapia, J.C. Protein Kinase CK2 in Cancer Energetics. Front. Oncol. 2020, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Rowse, A.L.; Gibson, S.A.; Meares, G.P.; Rajbhandari, R.; Nozell, S.E.; Dees, K.J.; Hjelmeland, A.B.; McFarland, B.C.; Benveniste, E.N. Protein Kinase CK2 Is Important for the Function of Glioblastoma Brain Tumor Initiating Cells. J. Neurooncol. 2017, 132, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Drug Delivery Approaches for the Treatment of Glioblastoma Multiforme. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.S.; Lee, B.-S.; Zhang, M.; Yu, J.S. Nanotechnology for Treatment of Glioblastoma Multiforme. J. Transl. Int. Med. 2018, 6, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Borgo, C.; D’Amore, C.; Sarno, S.; Salvi, M.; Ruzzene, M. Protein Kinase CK2: A Potential Therapeutic Target for Diverse Human Diseases. Signal Transduct. Target. Ther. 2021, 6, 183. [Google Scholar] [CrossRef]
- Ji, H.; Lu, Z. The Role of Protein Kinase CK2 in Glioblastoma Development. Clin. Cancer Res. 2013, 19, 6335–6337. [Google Scholar] [CrossRef]
- Singh, N.N.; Ramji, D.P. Protein Kinase CK2, an Important Regulator of the Inflammatory Response? J. Mol. Med. 2008, 86, 887–897. [Google Scholar] [CrossRef]
- Gibson, S.A.; Benveniste, E.N. Protein Kinase CK2: An Emerging Regulator of Immunity. Trends Immunol. 2018, 39, 82–85. [Google Scholar] [CrossRef]
- Ka, S.-O.; Hwang, H.P.; Jang, J.-H.; Hyuk Bang, I.; Bae, U.-J.; Yu, H.C.; Cho, B.H.; Park, B.-H. The Protein Kinase 2 Inhibitor Tetrabromobenzotriazole Protects against Renal Ischemia Reperfusion Injury. Sci. Rep. 2015, 5, 14816. [Google Scholar] [CrossRef]
- Apopa, P.L.; He, X.; Ma, Q. Phosphorylation of Nrf2 in the Transcription Activation Domain by Casein Kinase 2 (CK2) Is Critical for the Nuclear Translocation and Transcription Activation Function of Nrf2 in IMR-32 Neuroblastoma Cells. J. Biochem. Mol. Toxicol 2008, 22, 63–76. [Google Scholar] [CrossRef]
- Pi, J.; Bai, Y.; Reece, J.M.; Williams, J.; Liu, D.; Freeman, M.L.; Fahl, W.E.; Shugar, D.; Liu, J.; Qu, W. Molecular Mechanism of Human Nrf2 Activation and Degradation: Role of Sequential Phosphorylation by Protein Kinase CK2. Free Radic. Biol. Med. 2007, 42, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.A.; Meggio, F.; Ruzzene, M.; Andrzejewska, M.; Kazimierczuk, Z.; Pinna, L.A. 2-Dimethylamino-4,5,6,7-Tetrabromo-1H-Benzimidazole: A Novel Powerful and Selective Inhibitor of Protein Kinase CK2. Biochem. Biophys. Res. Commun. 2004, 321, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Battistutta, R.; Mazzorana, M.; Sarno, S.; Kazimierczuk, Z.; Zanotti, G.; Pinna, L.A. Inspecting the Structure-Activity Relationship of Protein Kinase CK2 Inhibitors Derived from Tetrabromo-Benzimidazole. Chem. Biol. 2005, 12, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Łukowska-Chojnacka, E.; Wińska, P.; Wielechowska, M.; Poprzeczko, M.; Bretner, M. Synthesis of Novel Polybrominated Benzimidazole Derivatives—Potential CK2 Inhibitors with Anticancer and Proapoptotic Activity. Bioorg. Med. Chem. 2016, 24, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Alberti, S.; Gaggero, G.; Ferretti, M.; Botter, R.; Vicini, S.; Castellano, M. An Up-to-Date Review on Alginate Nanoparticles and Nanofibers for Biomedical and Pharmaceutical Applications. Adv. Mater. Interfaces 2021, 8, 2100809. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, B.; Maji, R. Calcium Alginate/Gum Arabic Beads Containing Glibenclamide: Development and In Vitro Characterization. Int. J. Biol. Macromol. 2012, 51, 1070–1078. [Google Scholar] [CrossRef]
- Bhujbal, S.V.; Paredes-Juarez, G.A.; Niclou, S.P.; de Vos, P. Factors Influencing the Mechanical Stability of Alginate Beads Applicable for Immunoisolation of Mammalian Cells. J. Mech. Behav. Biomed. Mater. 2014, 37, 196–208. [Google Scholar] [CrossRef]
- Moradhaseli, S.; Sarzaeem, A.; Mirakabadi, A.Z.; Mohammadpour Dounighi, N.; Soheily, S.; Borumand, M.R. Preparation and Characterization of Sodium Alginate Nanoparticles Containing ICD-85 (Venom Derived Peptides). Int. J. Innov. Appl. Stud. 2013, 4, 534–542. [Google Scholar]
- Dounighi, N.; Zolfagharian, H.; Khaki, P.; Bidhendi, S.; Sarei, F. Alginate Nanoparticles as a Promising Adjuvant and Vaccine Delivery System. Indian J. Pharm. Sci. 2013, 75, 442. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Singh, S.K.; Kim, S.-K. Preparations and Applications of Alginate Nanoparticles. In Seaweed Polysaccharides; Elsevier: Amsterdam, The Netherlands, 2017; pp. 251–268. [Google Scholar]
- Thai, H.; Thuy Nguyen, C.; Thi Thach, L.; Thi Tran, M.; Duc Mai, H.; Thi Thu Nguyen, T.; Duc Le, G.; Van Can, M.; Dai Tran, L.; Long Bach, G.; et al. Characterization of Chitosan/Alginate/Lovastatin Nanoparticles and Investigation of Their Toxic Effects In Vitro and In Vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and Characterization of Calcium Alginate Nanoparticles, Sodium Homopolymannuronate Salt and Its Calcium Nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Adrian, E.; Treľová, D.; Filová, E.; Kumorek, M.; Lobaz, V.; Poreba, R.; Janoušková, O.; Pop-Georgievski, O.; Lacík, I.; Kubies, D. Complexation of CXCL12, FGF-2 and VEGF with Heparin Modulates the Protein Release from Alginate Microbeads. Int. J. Mol. Sci. 2021, 22, 11666. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, N.; Jouyban, A. Review of Pharmaceutical Applications of Diethylene Glycol Monoethyl Ether. J. Pharm. Pharm. Sci. 2022, 25, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Tiwary, A.K.; Bedi, N. Canagliflozin Loaded SMEDDS: Formulation Optimization for Improved Solubility, Permeability and Pharmacokinetic Performance. J. Pharm. Investig. 2019, 49, 67–85. [Google Scholar] [CrossRef]
- Alvi, M.M.; Chatterjee, P. A Prospective Analysis of Co-Processed Non-Ionic Surfactants in Enhancing Permeability of a Model Hydrophilic Drug. AAPS PharmSciTech 2014, 15, 339–353. [Google Scholar] [CrossRef]
- Luo, Y.; Hong, Y.; Shen, L.; Wu, F.; Lin, X. Multifunctional Role of Polyvinylpyrrolidone in Pharmaceutical Formulations. AAPS PharmSciTech 2021, 22, 34. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in Nanoparticle Synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Vas, A.; Árvai, G.; Fenyvesi, É.; Jicsinszky, L.; Budai, I.; Bényei, A.; Regdon, G.; Rusznyák, Á.; Vasvári, G.; et al. Investigation of the Drug Carrier Properties of Insoluble Cyclodextrin Polymer Microspheres. Biomolecules 2022, 12, 931. [Google Scholar] [CrossRef]
- Amini, Y.; Amel Jamehdar, S.; Sadri, K.; Zare, S.; Musavi, D.; Tafaghodi, M. Different Methods to Determine the Encapsulation Efficiency of Protein in PLGA Nanoparticles. Biomed. Mater. Eng. 2017, 28, 613–620. [Google Scholar] [CrossRef]
- Manjanna, K.M.; Pramod Kumar, T.M.; Shivakumar, B. Calcium Alginate Cross-Linked Polymeric Microbeads for Oral Sustained Drug Delivery in Arthritis. Drug Discov. Ther. 2010, 4, 109–122. [Google Scholar] [PubMed]
- Mallikarjuna Setty, C.; Sahoo, S.S.; Sa, B. Alginate-Coated Alginate-Polyethyleneimine Beads for Prolonged Release of Furosemide in Simulated Intestinal Fluid. Drug Dev. Ind. Pharm. 2005, 31, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.R.; Aguilar, E.; Hurtado, M. Dissolution behavior of carbamazepine suspensions using the usp dissolution apparatus 2 and the flow-through cell method with simulated gi fluids. Int. J. Pharm. Pharm. Sci. 2017, 9, 111. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. SLAS Technol. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus Religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Jabor Gozzi, G.; Bouaziz, Z.; Winter, E.; Daflon-Yunes, N.; Aichele, D.; Nacereddine, A.; Marminon, C.; Valdameri, G.; Zeinyeh, W.; Bollacke, A.; et al. Converting Potent Indeno [1,2-b]Indole Inhibitors of Protein Kinase CK2 into Selective Inhibitors of the Breast Cancer Resistance Protein ABCG2. J. Med. Chem. 2015, 58, 265–277. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity, German version EN ISO 10993-5:2009. ISO: Geneva, Switzerland, 2009.
- Pagano, M.A.; Bain, J.; Kazimierczuk, Z.; Sarno, S.; Ruzzene, M.; Di Maira, G.; Elliott, M.; Orzeszko, A.; Cozza, G.; Meggio, F.; et al. The Selectivity of Inhibitors of Protein Kinase CK2: An Update. Biochem. J. 2008, 415, 353–365. [Google Scholar] [CrossRef]
- Prudent, R.; Moucadel, V.; Nguyen, C.-H.; Barette, C.; Schmidt, F.; Florent, J.-C.; Lafanechère, L.; Sautel, C.F.; Duchemin-Pelletier, E.; Spreux, E.; et al. Antitumor Activity of Pyridocarbazole and Benzopyridoindole Derivatives That Inhibit Protein Kinase CK2. Cancer Res. 2010, 70, 9865–9874. [Google Scholar] [CrossRef]
- Zheng, Y.; McFarland, B.C.; Drygin, D.; Yu, H.; Bellis, S.L.; Kim, H.; Bredel, M.; Benveniste, E.N. Targeting Protein Kinase CK2 Suppresses Prosurvival Signaling Pathways and Growth of Glioblastoma. Clin. Cancer Res. 2013, 19, 6484–6494. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, D.; Yan, Z.; Zhang, Z.; Zheng, Y.; Zhang, Y.; Xue, C. Preparation and Characterization of the Ag2Se Flexible Films Tuned by PVP for Wearable Thermoelectric Generator. J. Mater. Sci. Mater. Electron. 2021, 32, 20295–20305. [Google Scholar] [CrossRef]
- McDonald, B.F.; Coulter, I.S.; Marison, I.W. Microbeads: A Novel Multiparticulate Drug Delivery Technology for Increasing the Solubility and Dissolution of Celecoxib. Pharm. Dev. Technol. 2015, 20, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Cerea, M.; Pattarino, F.; Foglio Bonda, A.; Palugan, L.; Segale, L.; Vecchio, C. Preparation of Multiparticulate Systems for Oral Delivery of a Micronized or Nanosized Poorly Soluble Drug. Drug Dev. Ind. Pharm. 2016, 42, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Kósa, D.; Pető, Á.; Fenyvesi, F.; Váradi, J.; Vecsernyés, M.; Budai, I.; Németh, J.; Fehér, P.; Bácskay, I.; Ujhelyi, Z. Oral Bioavailability Enhancement of Melanin Concentrating Hormone, Development and In Vitro Pharmaceutical Assessment of Novel Delivery Systems. Pharmaceutics 2021, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Pal, D. Development of PH-Sensitive Tamarind Seed Polysaccharide–Alginate Composite Beads for Controlled Diclofenac Sodium Delivery Using Response Surface Methodology. Int. J. Biol. Macromol. 2011, 49, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel Solid Self-Nanoemulsifying Drug Delivery System (S-SNEDDS) for Oral Delivery of Olmesartan Medoxomil: Design, Formulation, Pharmacokinetic and Bioavailability Evaluation. Pharmaceutics 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the Human Colon Carcinoma Cell Line (Caco-2) as a Model System for Intestinal Epithelial Permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Seithel, A.; Karlsson, J.; Hilgendorf, C.; Björquist, A.; Ungell, A.-L. Variability in MRNA Expression of ABC- and SLC-Transporters in Human Intestinal Cells: Comparison between Human Segments and Caco-2 Cells. Eur. J. Pharm. Sci. 2006, 28, 291–299. [Google Scholar] [CrossRef]
- Joki, T.; Machluf, M.; Atala, A.; Zhu, J.; Seyfried, N.T.; Dunn, I.F.; Abe, T.; Carroll, R.S.; Black, P.M. Continuous Release of Endostatin from Microencapsulated Engineered Cells for Tumor Therapy. Nat. Biotechnol. 2001, 19, 35–39. [Google Scholar] [CrossRef]
- Mangla, B.; Neupane, Y.R.; Singh, A.; Kumar, P.; Shafi, S.; Kohli, K. Lipid-Nanopotentiated Combinatorial Delivery of Tamoxifen and Sulforaphane: Ex Vivo, In Vivo and Toxicity Studies. Nanomedicine 2020, 15, 2563–2583. [Google Scholar] [CrossRef]
- Kaneda, Y.; Tsutsumi, Y.; Yoshioka, Y.; Kamada, H.; Yamamoto, Y.; Kodaira, H.; Tsunoda, S.; Okamoto, T.; Mukai, Y.; Shibata, H.; et al. The Use of PVP as a Polymeric Carrier to Improve the Plasma Half-Life of Drugs. Biomaterials 2004, 25, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Špaglová, M.; Čuchorová, M.; Šimunková, V.; Matúšová, D.; Čierna, M.; Starýchová, L.; Bauerová, K. Possibilities of the Microemulsion Use as Indomethacin Solubilizer and Its Effect on In Vitro and Ex Vivo Drug Permeation from Dermal Gels in Comparison with Transcutol®. Drug Dev. Ind. Pharm. 2020, 46, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Katsuma, S.; Adachi, T.; Hirasawa, A.; Shiojima, S.; Kadowaki, T.; Okuno, Y.; Koshimizu, T.-A.; Fujii, S.; Sekiya, Y.; et al. Inhibition of Protein Kinase CK2 Prevents the Progression of Glomerulonephritis. Proc. Natl. Acad. Sci. USA 2005, 102, 7736–7741. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Westerheide, S.D.; Hanson, J.L.; Baldwin, A.S. Tumor Necrosis Factor α-Induced Phosphorylation of RelA/P65 on Ser529 Is Controlled by Casein Kinase II. J. Biol. Chem. 2000, 275, 32592–32597. [Google Scholar] [CrossRef]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour Necrosis Factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Józsa, L.; Vasvári, G.; Sinka, D.; Nemes, D.; Ujhelyi, Z.; Vecsernyés, M.; Váradi, J.; Fenyvesi, F.; Lekli, I.; Gyöngyösi, A.; et al. Enhanced Antioxidant and Anti-Inflammatory Effects of Self-Nano and Microemulsifying Drug Delivery Systems Containing Curcumin. Molecules 2022, 27, 6652. [Google Scholar] [CrossRef]












| Entry | Composition | Excipient |
|---|---|---|
| 1 | CK2 inhib. beads | - |
| 2 | CK2 inhib. beads + TC | Transcutol® HP (0.01% v/v) |
| 3 | CK2 inhib. beads + PVP | PVP (2% w/v) |
| 4 | CK2 inhib. beads + TC + PVP | Transcutol® HP (0.01% v/v)PVP (2% w/v) |
| Diameter of the Nozzle [μm] | Vibration Frequency [Hz] | Electrostatic Voltage [V] | Flow Rate (mL/min) |
|---|---|---|---|
| 200 | 1800 | 1000–1200 | 5.06 |
| Entry | Composition | Particle Size (µm) | PDI |
|---|---|---|---|
| 1 | CK2 inhib. (DMAT) beads | 272.62 ± 10.03 | 0.40 ± 0.03 |
| 2 | CK2 inhib. beads+ TC | 279.67 ± 10.49 | 0.38 ± 0.02 |
| 3 | CK2 inhib. beads + PVP | 288.91 ± 5.28 | 0.24 ± 0.01 |
| 4 | CK2 inhib. beads + TC + PVP | 294.83 ± 8.46 | 0.33 ± 0.04 |
| Entry | Composition a | EE (%) b |
|---|---|---|
| 1 | CK2 inhib. (DMAT) beads | 64.32 ± 0.72 |
| 2 | CK2 inhib. beads + TC | 70.12 ± 0.81 |
| 3 | CK2 inhib. beads + PVP | 72.35 ± 0.66 |
| 4 | CK2 inhib. beads + TC+ PVP | 84.07 ± 1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papp, B.; Le Borgne, M.; Perret, F.; Marminon, C.; Józsa, L.; Pető, Á.; Kósa, D.; Nagy, L.; Kéki, S.; Ujhelyi, Z.; et al. Formulation and Investigation of CK2 Inhibitor-Loaded Alginate Microbeads with Different Excipients. Pharmaceutics 2023, 15, 2701. https://doi.org/10.3390/pharmaceutics15122701
Papp B, Le Borgne M, Perret F, Marminon C, Józsa L, Pető Á, Kósa D, Nagy L, Kéki S, Ujhelyi Z, et al. Formulation and Investigation of CK2 Inhibitor-Loaded Alginate Microbeads with Different Excipients. Pharmaceutics. 2023; 15(12):2701. https://doi.org/10.3390/pharmaceutics15122701
Chicago/Turabian StylePapp, Boglárka, Marc Le Borgne, Florent Perret, Christelle Marminon, Liza Józsa, Ágota Pető, Dóra Kósa, Lajos Nagy, Sándor Kéki, Zoltán Ujhelyi, and et al. 2023. "Formulation and Investigation of CK2 Inhibitor-Loaded Alginate Microbeads with Different Excipients" Pharmaceutics 15, no. 12: 2701. https://doi.org/10.3390/pharmaceutics15122701
APA StylePapp, B., Le Borgne, M., Perret, F., Marminon, C., Józsa, L., Pető, Á., Kósa, D., Nagy, L., Kéki, S., Ujhelyi, Z., Pallér, Á., Budai, I., Bácskay, I., & Fehér, P. (2023). Formulation and Investigation of CK2 Inhibitor-Loaded Alginate Microbeads with Different Excipients. Pharmaceutics, 15(12), 2701. https://doi.org/10.3390/pharmaceutics15122701