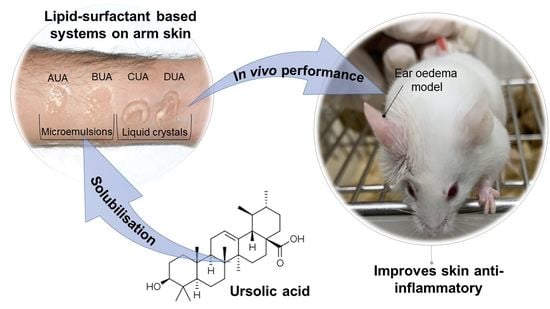
Design and Characterization of Lipid-Surfactant-Based Systems for Enhancing Topical Anti-Inflammatory Activity of Ursolic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ternary Phase Diagram
2.3. Selected Formulations and Incorporation of Ursolic Acid
2.4. Polarized Light Microscopy
2.5. Flow Rheology
2.6. Oscillatory Rheology
2.7. Texture Profile Analysis
2.8. Bioadhesion Studies
2.9. Croton-Oil-Induced Ear Edema
2.9.1. Animals
2.9.2. Treatments
2.9.3. Edema Induced by Croton Oil
2.10. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Frighetto, R.T.; Welendorf, R.M.; Nigro, E.N.; Frighetto, N.; Siani, A.C. Isolation of ursolic acid from apple peels by high speed counter-current chromatography. Food Chem. 2008, 106, 767–771. [Google Scholar] [CrossRef]
- Butkevičiūtė, A.; Liaudanskas, M.; Kviklys, D.; Zymonė, K.; Raudonis, R.; Viškelis, J.; Uselis, N.; Janulis, V. Detection and analysis of triterpenic compounds in apple extracts. Int. J. Food Prop. 2018, 21, 1716–1727. [Google Scholar] [CrossRef] [Green Version]
- Zou, S.; Chen, W. Determination of oleanolic and ursolic acids in different parts of Perilla frutescens by high-performance liquid chromatography. J. Braz. Chem. Soc. 2008, 19, 1429–1432. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.T.; Ho, C.T.; Wang, Z.Y.; Ferraro, T.; Lou, Y.R.; Stauber, K.; Ma, W.; Georgiadis, C.; Laskin, J.D.; Conney, A.H. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994, 54, 701–708. [Google Scholar] [PubMed]
- Lee, S.Y.; Kim, Y.J.; Chung, S.O.; Park, S.U. Recent studies on ursolic acid and its biological and pharmacological activity. EXCLI J. 2016, 15, 221–228. [Google Scholar] [CrossRef]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Ali, S.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Islam, A.; Hassan, I.; Yadav, D.K. Therapeutic Potential of Ursolic Acid in Cancer and Diabetic Neuropathy Diseases. Int. J. Mol. Sci. 2021, 22, 12162. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Kalinowska, M.; Gryko, K. Enhanced Antioxidant Activity of Ursolic Acid by Complexation with Copper (II): Experimental and Theoretical Study. Materials 2021, 14, 264. [Google Scholar] [CrossRef]
- Pironi, A.M.; De Araújo, P.R.; Fernandes, M.A.; Salgado, H.R.N.; Chorilli, M. Characteristics, Biological Properties and Analytical Methods of Ursolic Acid: A Review. Crit. Rev. Anal. Chem. 2018, 48, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, X.; Ding, J.; Xu, H.; Dai, X.; Hou, Z.; Zhang, K.; Sun, K.; Sun, W. Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int. J. Pharm. 2013, 441, 261–268. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Liu, Y.; Wang, Q.; Xing, S.; Li, L.; Wang, L.; Liu, L.; Gao, D. Ursolic acid liposomes with chitosan modification: Promising antitumor drug delivery and efficacy. Mater. Sci. Eng. C 2017, 71, 1231–1240. [Google Scholar] [CrossRef]
- Zhou, M.; Yi, Y.; Liu, L.; Lin, Y.; Li, J.; Ruan, J.; Zhong, Z. Polymeric micelles loading with ursolic acid enhancing anti-tumor effect on hepatocellular carcinoma. J. Cancer 2019, 10, 5820–5831. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, S.; De, A.K.; Bera, T. Oral delivery of ursolic acid-loaded nanostructured lipid carrier coated with chitosan oligosaccharides: Development, characterization, in vitro and in vivo assessment for the therapy of leishmaniasis. Int. J. Biol. Macromol. 2017, 102, 996–1008. [Google Scholar] [CrossRef]
- Barry, B.W. Drug delivery routes in skin: A novel approach. Adv. Drug Deliv. Rev. 2002, 54, S31–S40. [Google Scholar] [CrossRef]
- Drummond, C.J.; Fong, C. Surfactant self-assembly objects as novel drug delivery vehicles. Curr. Opin. Colloid Interface Sci. 1999, 4, 449–456. [Google Scholar] [CrossRef]
- Hamed, R.; Abu Kwiak, A.D.; Al-Adhami, Y.; Hammad, A.M.; Obaidat, R.; Abusara, O.H.; Abu Huwaij, R. Microemulsions as Lipid Nanosystems Loaded into Thermoresponsive In Situ Microgels for Local Ocular Delivery of Prednisolone. Pharmaceutics 2022, 14, 1975. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Solans, C.; García-Celma, M.J. Surfactants for microemulsions. Curr. Opin. Colloid Interface Sci. 1997, 2, 464–471. [Google Scholar] [CrossRef]
- Garti, N.; Libster, D.; Aserin, A. Lipid polymorphism in lyotropic liquid crystals for triggered release of bioactives. Food Funct. 2012, 3, 700–713. [Google Scholar] [CrossRef]
- Martiel, I.; Baumann, N.; Vallooran, J.J.; Bergfreund, J.; Sagalowicz, L.; Mezzenga, R. Oil and drug control the release rate from lyotropic liquid crystals. J. Control. Release 2015, 204, 78–84. [Google Scholar] [CrossRef]
- Milak, S.; Zimmer, A. Glycerol monooleate liquid crystalline phases used in drug delivery systems. Int. J. Pharm. 2015, 478, 569–587. [Google Scholar] [CrossRef] [PubMed]
- de Souza, I.F.F.; dos Santos, T.Q.; Placido, R.V.; Mangerona, B.A.; Carvalho, F.C.; Boralli, V.B.; Ruela, A.L.M.; Pereira, G.R. The liquid crystalline phase behaviour of a nasal formulation modifies the brain disposition of donepezil in rats in the treatment of Alzheimer’s disease. Colloids Surf. B Biointerfaces 2021, 203, 111721. [Google Scholar] [CrossRef]
- Aida, K.L.; Kreling, P.F.; Caiaffa, K.S.; Calixto, G.M.F.; Chorilli, M.; Spolidorio, D.M.; Santos-Filho, N.A.; Cilli, E.M.; Duque, C. Antimicrobial peptide-loaded liquid crystalline precursor bioadhesive system for the prevention of dental caries. Int. J. Nanomed. 2018, 13, 3081–3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, M.A.D.S.; Calixto, G.M.F.; de Toledo, L.G.; Bonifácio, B.V.; dos Santos, L.C.; de Almeida, M.T.G.; Chorilli, M.; Bauab, T.M. Liquid crystal precursor mucoadhesive system as a strategy to improve the prophylactic action of Syngonanthus nitens (Bong.) Ruhland against infection by Candida krusei. Int. J. Nanomed. 2015, 10, 7455–7466. [Google Scholar] [CrossRef] [PubMed]
- Gonçalez, M.L.; Corrêa, M.A.; Chorilli, M. Skin Delivery of Kojic Acid-Loaded Nanotechnology-Based Drug Delivery Systems for the Treatment of Skin Aging. BioMed Res. Int. 2013, 2013, 271276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Wang, J.; Cao, F.; Lee, R.J.; Zhai, G. Lyotropic liquid crystal systems in drug delivery. Drug Discov. Today 2010, 15, 1032–1040. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Gui, S.; Huang, J.; Cao, J.; Li, Z.; Li, Q.; Chu, X. Characterization of Lipid-Based Lyotropic Liquid Crystal and Effects of Guest Molecules on Its Microstructure: A Systematic Review. AAPS PharmSciTech 2018, 19, 2023–2040. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, P.; Gui, S. Cubic and Hexagonal Liquid Crystals as Drug Delivery Systems. BioMed Res. Int. 2014, 2014, 815981. [Google Scholar] [CrossRef] [Green Version]
- Rajabalaya, R.; Musa, M.N.; Kifli, N.; David, S.R. Oral and transdermal drug delivery systems: Role of lipid-based lyotropic liquid crystals. Drug Des. Dev. Ther. 2017, 11, 393–406. [Google Scholar] [CrossRef] [Green Version]
- Shah, J.C. Cubic phase gels as drug delivery systems. Adv. Drug Deliv. Rev. 2001, 47, 229–250. [Google Scholar] [CrossRef]
- Segre, J.A. Epidermal barrier formation and recovery in skin disorders. J. Clin. Investig. 2006, 116, 1150–1158. [Google Scholar] [CrossRef]
- Halder, R.M.; Nootheti, P.K. Ethnic skin disorders overview. J. Am. Acad. Dermatol. 2003, 48, S143–S148. [Google Scholar] [CrossRef]
- Gladman, A.C. Toxicodendron Dermatitis: Poison Ivy, Oak, and Sumac. Wilderness Environ. Med. 2006, 17, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2009, 124, R7–R12. [Google Scholar] [CrossRef]
- Luger, T.; Amagai, M.; Dreno, B.; Dagnelie, M.-A.; Liao, W.; Kabashima, K.; Schikowski, T.; Proksch, E.; Elias, P.M.; Simon, M.; et al. Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. J. Dermatol. Sci. 2021, 102, 142–157. [Google Scholar] [CrossRef]
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021, 9, 682414. [Google Scholar] [CrossRef]
- Huzil, J.T.; Sivaloganathan, S.; Kohandel, M.; Foldvari, M. Drug delivery through the skin: Molecular simulations of barrier lipids to design more effective noninvasive dermal and transdermal delivery systems for small molecules, biologics, and cosmetics. WIREs Nanomed. Nanobiotechnol. 2011, 3, 449–462. [Google Scholar] [CrossRef]
- Dick, I.P.; Scott, R.C. Pig Ear Skin as an In-vitro Model for Human Skin Permeability. J. Pharm. Pharmacol. 1992, 44, 640–645. [Google Scholar] [CrossRef]
- Santos, A.D.J.; Macêdo, N.A.; Cavalcanti, S.C.D.H.; Sarmento, V.H.V.; Lira, A.A.M.; dos Santos, C.P.; Santos, R.L.C.; Nunes, R.D.S. Larvicidal formulation containing N-tosylindole: A viable alternative to chemical control of Aedes aegypti. Colloids Surf. B Biointerfaces 2022, 213, 112380. [Google Scholar] [CrossRef]
- Kralova, I.; Sjöblom, J. Surfactants Used in Food Industry: A Review. J. Dispers. Sci. Technol. 2009, 30, 1363–1383. [Google Scholar] [CrossRef]
- Salikolimi, K.; Sudhakar, A.A.; Ishida, Y. Functional Ionic Liquid Crystals. Langmuir 2020, 36, 11702–11731. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Bonifácio, B.V.; Baub, T.M.; Gremião, M.P.D.; Chorilli, M. In-Situ Gelling Liquid Crystal Mucoadhesive Vehicle for Curcumin Buccal Administration and Its Potential Application in the Treatment of Oral Candidiasis. J. Biomed. Nanotechnol. 2019, 15, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Souto, D.E.; Fonseca, A.M.; Barragan, J.T.; Luz, R.D.C.; Andrade, H.M.; Damos, F.S.; Kubota, L.T. SPR analysis of the interaction between a recombinant protein of unknown function in Leishmania infantum immobilised on dendrimers and antibodies of the visceral leishmaniasis: A potential use in immunodiagnosis. Biosens. Bioelectron. 2015, 70, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Benigni, M.; Pescina, S.; Grimaudo, M.A.; Padula, C.; Santi, P.; Nicoli, S. Development of microemulsions of suitable viscosity for cyclosporine skin delivery. Int. J. Pharm. 2018, 545, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Chorilli, M.; Prestes, P.S.; Rigon, R.B.; Leonardi, G.R.; Chiavacci, L.A.; Sarmento, V.H.V.; Oliveira, A.G.; Scarpa, M.V. Structural characterization and in vivo evaluation of retinyl palmitate in non-ionic lamellar liquid crystalline system. Colloids Surf. B Biointerfaces 2011, 85, 182–188. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; dos Santos, A.M.; Rodero, C.F.; Gremião, M.P.D.; Chorilli, M. Design, characterization, and biological evaluation of curcumin-loaded surfactant-based systems for topical drug delivery. Int. J. Nanomed. 2016, 11, 4553–4562. [Google Scholar] [CrossRef] [Green Version]
- Fonseca-Santos, B.; Satake, C.Y.; Calixto, G.M.F.; dos Santos, A.M.; Chorilli, M. Trans-resveratrol-loaded nonionic lamellar liquid-crystalline systems: Structural, rheological, mechanical, textural, and bioadhesive characterization and evaluation of in vivo anti-inflammatory activity. Int. J. Nanomed. 2017, 12, 6883–6893. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, A.T.; Martinez, R.M.; Pinho-Ribeiro, F.A.; da Silva, A.M.L.D.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Chorilli, M.; Casagrande, R. Resveratrol-Loaded Liquid-Crystalline System Inhibits UVB-Induced Skin Inflammation and Oxidative Stress in Mice. J. Nat. Prod. 2016, 79, 1329–1338. [Google Scholar] [CrossRef]
- Naoui, W.; Bolzinger, M.-A.; Fenet, B.; Pelletier, J.; Valour, J.-P.; Kalfat, R.; Chevalier, Y. Microemulsion Microstructure Influences the Skin Delivery of an Hydrophilic Drug. Pharm. Res. 2011, 28, 1683–1695. [Google Scholar] [CrossRef]
- Huang, Y.; Gui, S. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. RSC Adv. 2018, 8, 6978–6987. [Google Scholar] [CrossRef] [Green Version]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Fong, C.; Le, T.; Drummond, C.J. Lyotropic liquid crystal engineering–ordered nanostructured small molecule amphiphile self-assembly materials by design. Chem. Soc. Rev. 2012, 41, 1297–1322. [Google Scholar] [CrossRef]
- Manaia, E.B.; Abuçafy, M.P.; Chiari-Andréo, B.G.; Silva, B.L.; Oshiro-Júnior, J.A.; Chiavacci, L.A. Physicochemical characterization of drug nanocarriers. Int. J. Nanomed. 2017, 12, 4991–5011. [Google Scholar] [CrossRef] [Green Version]
- Badawi, A.A.; Nour, S.A.; Sakran, W.S.; El-Mancy, S.M.S. Preparation and Evaluation of Microemulsion Systems Containing Salicylic Acid. AAPS PharmSciTech 2009, 10, 1081–1084. [Google Scholar] [CrossRef] [Green Version]
- Szlezak, M.; Nieciecka, D.; Joniec, A.; Pękała, M.; Gorecka, E.; Emo, M.; Stébé, M.J.; Krysiński, P.; Bilewicz, R. Monoolein Cubic Phase Gels and Cubosomes Doped with Magnetic Nanoparticles–Hybrid Materials for Controlled Drug Release. ACS Appl. Mater. Interfaces 2017, 9, 2796–2805. [Google Scholar] [CrossRef]
- Ryklin, I.; Byers, B. Shear-Thinning Lamellar Gel Network Emulsions as Delivery Systems. In Delivery System Handbook for Personal Care and Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2005; pp. 547–568. [Google Scholar]
- Kwak, M.-S.; Ahn, H.-J.; Song, K.-W. Rheological investigation of body cream and body lotion in actual application conditions. Korea Aust. Rheol. J. 2015, 27, 241–251. [Google Scholar] [CrossRef]
- Gulão, E.D.S.; De Souza, C.J.F.; Da Costa, A.R.; Da Rocha-Leão, M.H.M.; Garcia-Rojas, E.E. Stability and rheological behavior of coconut oil-in-water emulsions formed by biopolymers. Polímeros 2018, 28, 413–421. [Google Scholar] [CrossRef]
- Otsubo, Y.; Prud’Homme, R.K. Flow behavior of oil-in-water emulsions. J. Rheol. 1993, 37, 561. [Google Scholar] [CrossRef] [Green Version]
- Resende, K.X.; Corrêa, M.A.; De Oliveira, A.G.; Scarpa, M.V. Effect of cosurfactant on the supramolecular structure and physicochemical properties of non-ionic biocompatible microemulsions. Rev. Bras. Ciências Farm. 2008, 44, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.B.; Calixto, G.; Graminha, M.; Cerecetto, H.; González, M.; Chorilli, M. Development, Characterization, andIn VitroBiological Performance of Fluconazole-Loaded Microemulsions for the Topical Treatment of Cutaneous Leishmaniasis. BioMed Res. Int. 2015, 2015, 396894. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.D.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Rigon, C.; Marchiori, M.C.L.; Jardim, F.D.S.; Pegoraro, N.S.; Chaves, P.D.S.; Velho, M.C.; Beck, R.C.R.; Ourique, A.; Sari, M.H.M.; de Oliveira, S.M.; et al. Hydrogel containing silibinin nanocapsules presents effective anti-inflammatory action in a model of irritant contact dermatitis in mice. Eur. J. Pharm. Sci. 2019, 137, 104969. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, P.R.; Calixto, G.M.F.; Silva, I.; Zago, L.H.D.P.; Junior, J.A.O.; Pavan, F.; Ribeiro, A.O.; Fontana, C.R.; Chorilli, M. Mucoadhesive In Situ Gelling Liquid Crystalline Precursor System to Improve the Vaginal Administration of Drugs. AAPS PharmSciTech 2019, 20, 225. [Google Scholar] [CrossRef] [PubMed]
- Rodero, C.F.; Calixto, G.M.F.; Dos Santos, K.C.; Sato, M.; Ramos, M.A.D.S.; Miró, M.S.; Rodríguez, E.; Vigezzi, C.; Bauab, T.M.; Sotomayor, C.E.; et al. Curcumin-Loaded Liquid Crystalline Systems for Controlled Drug Release and Improved Treatment of Vulvovaginal Candidiasis. Mol. Pharm. 2018, 15, 4491–4504. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Pacheco, C.D.N.; Pinto, M.C.; Chorilli, M. An effective mosquito-repellent topical product from liquid crystal-based tea tree oil. Ind. Crops Prod. 2019, 128, 488–495. [Google Scholar] [CrossRef]
- Hao, Z.-Q.; Chen, Z.-J.; Chang, M.-C.; Meng, J.-L.; Liu, J.-Y.; Feng, C.-P. Rheological properties and gel characteristics of polysaccharides from fruit-bodies of Sparassis crispa. Int. J. Food Prop. 2018, 21, 2283–2295. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.; Pathak, M.; Khan, M.K. Thermorheological Characterization of Elastoviscoplastic Carbopol Ultrez 20 Gel. J. Eng. Mater. Technol. 2015, 137, 031002. [Google Scholar] [CrossRef]
- Ghica, M.V.; Hîrjău, M.; Lupuleasa, D.; Dinu-Pîrvu, C.-E. Flow and Thixotropic Parameters for Rheological Characterization of Hydrogels. Molecules 2016, 21, 786. [Google Scholar] [CrossRef]
- Yu, T.; Malcolm, K.; Woolfson, D.; Jones, D.S.; Andrews, G.P. Vaginal gel drug delivery systems: Understanding rheological characteristics and performance. Expert Opin. Drug Deliv. 2011, 8, 1309–1322. [Google Scholar] [CrossRef]
- Torres, L.G.; Iturbe, R.; Snowden, M.J.; Chowdhry, B.Z.; Leharne, S.A. Preparation of o/w emulsions stabilized by solid particles and their characterization by oscillatory rheology. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 439–448. [Google Scholar] [CrossRef]
- Mezger, T. The Rheology Handbook: For Users of Rotational and Oscillator Y Rheometers, 5th ed.; Vincentz Network: Hannover, Germany, 2020. [Google Scholar]
- Tabilo-Munizaga, G.; Barbosa-Cánovas, G.V. Rheology for the food industry. J. Food Eng. 2005, 67, 147–156. [Google Scholar] [CrossRef]
- Saxena, A.; Kaloti, M.; Bohidar, H. Rheological properties of binary and ternary protein–polysaccharide co-hydrogels and comparative release kinetics of salbutamol sulphate from their matrices. Int. J. Biol. Macromol. 2011, 48, 263–270. [Google Scholar] [CrossRef]
- Maurer, S.; Junghans, A.; Vilgis, T.A. Impact of xanthan gum, sucrose and fructose on the viscoelastic properties of agarose hydrogels. Food Hydrocoll. 2012, 29, 298–307. [Google Scholar] [CrossRef]
- Sharma, A.; Rawat, K.; Solanki, P.R.; Aswal, V.; Kohlbrecher, J.; Bohidar, H. Internal structure and thermo-viscoelastic properties of agar ionogels. Carbohydr. Polym. 2015, 134, 617–626. [Google Scholar] [CrossRef]
- Singh, S.S.; Aswal, V.K.; Bohidar, H.B. Structural evolution of aging agar-gelatin co-hydrogels. Polymer 2009, 50, 5589–5597. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Campos, M.L.; Peccinini, R.G.; Gremião, M.P.D. Nasal administration of liquid crystal precursor mucoadhesive vehicle as an alternative antiretroviral therapy. Eur. J. Pharm. Biopharm. 2013, 84, 219–227. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Barbi, M.S.; Sarmento, V.H.V.; Chiavacci, L.A.; Netto, F.M.; Gremião, M.P. Surfactant systems for nasal zidovudine delivery: Structural, rheological and mucoadhesive properties. J. Pharm. Pharmacol. 2010, 62, 430–439. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Djokic, J. Texture profile analysis of bioadhesive polymeric semisolids: Mechanical characterization and investigation of interactions between formulation components. J. Appl. Polym. Sci. 1996, 61, 2229–2234. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Textural analysis and flow rheometry of novel, bioadhesive antimicrobial oral gels. Pharm. Res. 1997, 14, 450–457. [Google Scholar] [CrossRef]
- Cardoso, V.M.d.O.; Cury, B.S.F.; Evangelista, R.C.; Gremião, M.P.D. Development and characterization of cross-linked gellan gum and retrograded starch blend hydrogels for drug delivery applications. J. Mech. Behav. Biomed. Mater. 2017, 65, 317–333. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Kennedy, J.; Li, B.; Xu, X.; Xie, B. Characters of rice starch gel modified by gellan, carrageenan, and glucomannan: A texture profile analysis study. Carbohydr. Polym. 2007, 69, 411–418. [Google Scholar] [CrossRef]
- Bassi Da Silva, J.; Ferreira, S.B.D.S.; Reis, A.V.; Cook, M.T.; Bruschi, M.L. Assessing Mucoadhesion in Polymer Gels: The Effect of Method Type and Instrument Variables. Polymers 2018, 10, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevher, E.; Sensoy, D.; Taha, M.A.M.; Araman, A. Effect of Thiolated Polymers to Textural and Mucoadhesive Properties of Vaginal Gel Formulations Prepared with Polycarbophil and Chitosan. AAPS PharmSciTech 2008, 9, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, E.A. Rheology and sensory texture of biopolymer gels. Curr. Opin. Colloid Interface Sci. 2007, 12, 242–250. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Conceição, J.; Amaral, M.H.; Santos, D.; Silva, J.B.; Lobo, J.M.S. Characterization and stability studies of emulsion systems containing pumice. Braz. J. Pharm. Sci. 2014, 50, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Palacio, M.L.B.; Bhushan, B. Bioadhesion: A review of concepts and applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 2321–2347. [Google Scholar] [CrossRef]
- Parente, M.E.; Andrade, A.O.; Ares, G.; Russo, F.; Jiménez-Kairuz, Á. Bioadhesive hydrogels for cosmetic applications. Int. J. Cosmet. Sci. 2015, 37, 511–518. [Google Scholar] [CrossRef]
- Da Silva, P.B.; Calixto, G.M.F.; Júnior, J.A.O.; Bombardelli, R.L.; Fonseca-Santos, B.; Rodero, C.F.; Chorilli, M. Structural Features and the Anti-Inflammatory Effect of Green Tea Extract-Loaded Liquid Crystalline Systems Intended for Skin Delivery. Polymers 2017, 9, 30. [Google Scholar] [CrossRef]
- Oyafuso, M.H.; Carvalho, F.C.; Takeshita, T.M.; De Souza, A.L.R.; Araújo, D.R.; Merino, V.; Gremião, M.P.D.; Chorilli, M. Development and In Vitro Evaluation of Lyotropic Liquid Crystals for the Controlled Release of Dexamethasone. Polymers 2017, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent Anti-Inflammatory Activity of Ursolic Acid, a Triterpenoid Antioxidant, Is Mediated through Suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef] [Green Version]
- Martínez, A.L.; González-Trujano, M.E.; Chávez, M.; Pellicer, F. Antinociceptive effectiveness of triterpenes from rosemary in visceral nociception. J. Ethnopharmacol. 2012, 142, 28–34. [Google Scholar] [CrossRef]
- Tapondjou, L.A.; Lontsi, D.; Luc, S.B.; JongWon, C.; Kyung-Tae, L.; Hyun-Ju, J.; Hee-Juhn, P. In Vivo anti-nociceptive and anti-inflammatory effect of the two triterpenes, ursolic acid and 23-hydroxyursolic acid, fromcussonia bancoensis. Arch. Pharmacal Res. 2003, 26, 143–146. [Google Scholar] [CrossRef]
- Verano, J.; González-Trujano, M.E.; Déciga-Campos, M.; Ventura-Martínez, R.; Pellicer, F. Ursolic acid from Agastache mexicana aerial parts produces antinociceptive activity involving TRPV1 receptors, cGMP and a serotonergic synergism. Pharmacol. Biochem. Behav. 2013, 110, 255–264. [Google Scholar] [CrossRef]
- Dante, M.D.C.L.; Borgheti-Cardoso, L.N.; Fantini, M.C.D.A.; Praça, F.S.G.; Medina, W.S.G.; Pierre, M.B.R.; Lara, M.G. Liquid Crystalline Systems Based on Glyceryl Monooleate and Penetration Enhancers for Skin Delivery of Celecoxib: Characterization, In Vitro Drug Release, and In Vivo Studies. J. Pharm. Sci. 2018, 107, 870–878. [Google Scholar] [CrossRef]




| Sample | Herschel–Bulkley Model | Power Law Model | Bioadhesive Measurement | |||||
|---|---|---|---|---|---|---|---|---|
| σo (Pa) | k (Pa.sn) | n | R2 Adjusted | S | n | R2 Adjusted | Force of Detachment (mN) | |
| A | - | 0.1469 | 0.9360 | 0.9999 | 0.0075 | 1.8735 | 0.9621 | 3.0 ± 1.0 |
| AAU | 1.0245 | 0.0890 | 1.0628 | 0.9991 | 0.0023 | 2.4691 | 0.7578 | 1.0 ± 1.5 |
| B | - | 0.2219 | 0.9501 | 1.0000 | 0.0010 | 3.0376 | 0.8452 | 4.0 ± 4.5 |
| BAU | - | 0.3777 | 0.9077 | 1.0000 | 1182.0945 | 0.2531 | 0.9348 | 3.0 ± 1.8 |
| C | 41.8661 | 0.0494 | 0.8606 | 0.9805 | 1239.1706 | 0.2676 | 0.9457 | 31.0 ± 6.4 |
| CAU | 52.0744 | 3.6331 | 0.6951 | 0.9991 | 581.3953 | 0.0808 | 0.8715 | 65.0 ± 18.5 |
| D | 15.3213 | 1.1716 | 0.8473 | 0.9971 | 575.5286 | 0.0625 | 0.9885 | 78.0 ± 7.7 |
| DAU | 6.5332 | 29.3480 | 0.3870 | 0.9928 | 1851.0950 | 0.2184 | 0.9704 | 33.0 ± 16.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca-Santos, B.; Araujo, G.A.; Ferreira, P.S.; Victorelli, F.D.; Pironi, A.M.; Araújo, V.H.S.; Carvalho, S.G.; Chorilli, M. Design and Characterization of Lipid-Surfactant-Based Systems for Enhancing Topical Anti-Inflammatory Activity of Ursolic Acid. Pharmaceutics 2023, 15, 366. https://doi.org/10.3390/pharmaceutics15020366
Fonseca-Santos B, Araujo GA, Ferreira PS, Victorelli FD, Pironi AM, Araújo VHS, Carvalho SG, Chorilli M. Design and Characterization of Lipid-Surfactant-Based Systems for Enhancing Topical Anti-Inflammatory Activity of Ursolic Acid. Pharmaceutics. 2023; 15(2):366. https://doi.org/10.3390/pharmaceutics15020366
Chicago/Turabian StyleFonseca-Santos, Bruno, Giovanna Angeli Araujo, Paula Scanavez Ferreira, Francesca Damiani Victorelli, Andressa Maria Pironi, Victor Hugo Sousa Araújo, Suzana Gonçalves Carvalho, and Marlus Chorilli. 2023. "Design and Characterization of Lipid-Surfactant-Based Systems for Enhancing Topical Anti-Inflammatory Activity of Ursolic Acid" Pharmaceutics 15, no. 2: 366. https://doi.org/10.3390/pharmaceutics15020366
APA StyleFonseca-Santos, B., Araujo, G. A., Ferreira, P. S., Victorelli, F. D., Pironi, A. M., Araújo, V. H. S., Carvalho, S. G., & Chorilli, M. (2023). Design and Characterization of Lipid-Surfactant-Based Systems for Enhancing Topical Anti-Inflammatory Activity of Ursolic Acid. Pharmaceutics, 15(2), 366. https://doi.org/10.3390/pharmaceutics15020366