Influence of the Volatility of Solvent on the Reproducibility of Droplet Formation in Pharmaceutical Inkjet Printing
Abstract
:1. Introduction
- Z—Z number
- Oh—Ohnesorge number
- We—Weber number
- γ—surface tension
- ρ—density
- η—dynamic viscosity
- uD—velocity of droplet at flight
- dN—diameter of nozzle
- dD—diameter of droplet
2. Materials and Methods
2.1. Preparation of Drug Solutions
2.2. Characterization of Drug Solutions and Analysis of the Inkjet Printability
2.3. Inkjet Printing Device with Droplet Formation Analyzation Setup
2.4. Investigation of Inkjet Printing Parameters
2.5. Investigations of Long-Term Droplet Formation
- t = 1 min,
- t = 4 min,
- t = 15 min and
- t = 30 min.
2.6. Statistical Analysis
3. Results and Discussion
3.1. Properties of Drug Solutions and Inkjet Printability
3.2. Inkjet Printing Parameters
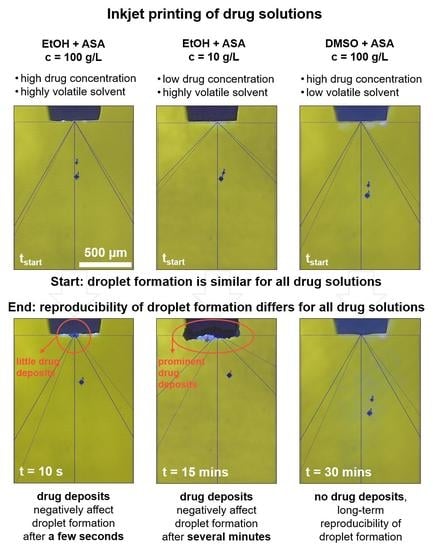
3.3. Long-Term Droplet Formation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alammar, A.; Kois, J.C.; Revilla-León, M.; Att, W. Additive Manufacturing Technologies: Current Status and Future Perspectives. J. Prosthodont. 2022, 31, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Dufaud, M.; Solé, L.; Maumus, M.; Simon, M.; Perrier-Groult, E.; Subra, G.; Jorgensen, C.; Noël, D. 3D bioprinting of articular cartilage: Recent advances and perspectives. Bioprinting 2022, 28, e00253. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Abbas, N.; Ali, A. Inkjet Printing: A Viable Technology for Biosensor Fabrication. Chemosensors 2022, 10, 103. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Zhang, L.; Xu, J.; Xiao, X.; Zhang, X. Inkjet-printed flexible sensors: From function materials, manufacture process, and applications perspective. Mater. Today Commun. 2022, 31, 103263. [Google Scholar] [CrossRef]
- Yang, X.; Lin, Y.; Wu, T.; Yan, Z.; Chen, Z.; Kuo, H.-C.; Zhang, R. An overview on the principle of inkjet printing technique and its application in micro-display for augmented/virtual realities. OEA 2022, 5, 210123. [Google Scholar] [CrossRef]
- Scoutaris, N.; Alexander, M.R.; Gellert, P.R.; Roberts, C.J. Inkjet printing as a novel medicine formulation technique. J. Control. Release 2011, 156, 179–185. [Google Scholar] [CrossRef]
- Genina, N.; Fors, D.; Palo, M.; Peltonen, J.; Sandler, N. Behavior of printable formulations of loperamide and caffeine on different substrates--effect of print density in inkjet printing. Int. J. Pharm. 2013, 453, 488–497. [Google Scholar] [CrossRef]
- Hirshfield, L.; Giridhar, A.; Taylor, L.S.; Harris, M.T.; Reklaitis, G.V. Dropwise Additive Manufacturing of Pharmaceutical Products for Solvent-Based Dosage Forms. J. Pharm. Sci. 2014, 103, 496–506. [Google Scholar] [CrossRef]
- Machekposhti, S.A.; Mohaved, S.; Narayan, R.J. Inkjet dispensing technologies: Recent advances for novel drug discovery. Expert Opin. Drug Discov. 2019, 14, 101–113. [Google Scholar] [CrossRef]
- Sen, K.; Manchanda, A.; Mehta, T.; Ma, A.W.K.; Chaudhuri, B. Formulation design for inkjet-based 3D printed tablets. Int. J. Pharm. 2020, 584, 119430. [Google Scholar] [CrossRef] [PubMed]
- Kreft, K.; Lavrič, Z.; Stanić, T.; Perhavec, P.; Dreu, R. Influence of the Binder Jetting Process Parameters and Binder Liquid Composition on the Relevant Attributes of 3D-Printed Tablets. Pharmaceutics 2022, 14, 1568. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, O.; Fischer, B.; Breitkreutz, J. Fundamental Investigations into Metoprolol Tartrate Deposition on Orodispersible Films by Inkjet Printing for Individualised Drug Dosing. Pharmaceutics 2021, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food & Drug Administration. SPRITAM (Levetiracetam) Tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207958Orig1s000TOC.cfm (accessed on 1 September 2022).
- Uddin, M.J.; Scoutaris, N.; Klepetsanis, P.; Chowdhry, B.; Prausnitz, M.R.; Douroumis, D. Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int. J. Pharm. 2015, 494, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Elahpour, N.; Pahlevanzadeh, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. 3D printed microneedles for transdermal drug delivery: A brief review of two decades. Int. J. Pharm. 2021, 597, 120301. [Google Scholar] [CrossRef]
- Scoutaris, N.; Chai, F.; Maurel, B.; Sobocinski, J.; Zhao, M.; Moffat, J.G.; Craig, D.Q.; Martel, B.; Blanchemain, N.; Douroumis, D. Development and Biological Evaluation of Inkjet Printed Drug Coatings on Intravascular Stent. Mol. Pharm. 2016, 13, 125–133. [Google Scholar] [CrossRef]
- Marizza, P.; Keller, S.S.; Boisen, A. Inkjet printing as a technique for filling of micro-wells with biocompatible polymers. Microelectron. Eng. 2013, 111, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Fox, C.B.; Nemeth, C.L.; Chevalier, R.W.; Cantlon, J.; Bogdanoff, D.B.; Hsiao, J.C.; Desai, T.A. Picoliter-volume inkjet printing into planar microdevice reservoirs for low-waste, high-capacity drug loading. Bioeng. Transl. Med. 2017, 2, 9–16. [Google Scholar] [CrossRef]
- Marizza, P.; Keller, S.S.; Müllertz, A.; Boisen, A. Polymer-filled microcontainers for oral delivery loaded using supercritical impregnation. J. Control. Release 2014, 173, 1–9. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, C.; Casielles, A.M.; Altay, A.; Bettini, R.; Alvarez-Lorenzo, C.; García-González, C.A. From the printer to the lungs: Inkjet-printed aerogel particles for pulmonary delivery. Chem. Eng. J. 2019, 357, 559–566. [Google Scholar] [CrossRef]
- Junqueira, L.A.; Tabriz, A.G.; Raposo, F.J.; Carobini, L.R.; Vaz, U.P.; Brandão, M.A.F.; Douroumis, D.; Raposo, N.R.B. Coupling of Fused Deposition Modeling and Inkjet Printing to Produce Drug Loaded 3D Printed Tablets. Pharmaceutics 2022, 14, 159. [Google Scholar] [CrossRef]
- Konasch, J.; Riess, A.; Mau, R.; Teske, M.; Rekowska, N.; Eickner, T.; Grabow, N.; Seitz, H. A Novel Hybrid Additive Manufacturing Process for Drug Delivery Systems with Locally Incorporated Drug Depots. Pharmaceutics 2019, 11, 661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scoutaris, N.; Ross, S.; Douroumis, D. Current Trends on Medical and Pharmaceutical Applications of Inkjet Printing Technology. Pharm. Res. 2016, 33, 1799–1816. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-H.; Gamboa, A.; Morales, J.O. Inkjet printing of small molecules, biologics, and nanoparticles. Int. J. Pharm. 2021, 600, 120462. [Google Scholar] [CrossRef]
- Liu, Y.; Derby, B. Experimental study of the parameters for stable drop-on-demand inkjet performance. Phys. Fluids 2019, 31, 32004. [Google Scholar] [CrossRef] [Green Version]
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet printing for pharmaceutics—A review of research and manufacturing. Int. J. Pharm. 2015, 494, 554–567. [Google Scholar] [CrossRef] [Green Version]
- Reis, N.; Derby, B. Ink jet deposition of ceramic suspensions: Modelling and experiments of droplet formation. Mater. Res. Soc. Symp. Proc. 2000, 625, 117–122. [Google Scholar] [CrossRef]
- Jang, D.; Kim, D.; Moon, J. Influence of fluid physical properties on ink-jet printability. Langmuir 2009, 25, 2629–2635. [Google Scholar] [CrossRef]
- Yarin, A.L. Drop Impact Dynamics: Splashing, Spreading, Receding, Bouncing. Annu. Rev. Fluid Mech. 2006, 38, 159–192. [Google Scholar] [CrossRef]
- Brock, T.; Groteklaes, M.; Mischke, P. Lehrbuch der Lacktechnologie; Vincentz: Hannover, Germany, 1998; ISBN 3878705476. [Google Scholar]
- Falbe, J.; Regitz, M.; Römpp, H. Römpp Chemie Lexikon: 3: H-L, 9th ed.; Thieme: Stuttgart, Germany, 1990; ISBN 3-13734-809-9. [Google Scholar]
- Vehse, M.; Gieseke, M.; Fiedler, S.; Petersen, S.; Irsig, R.; Senz, V.; Löbler, M.; Hustedt, M.; Kaierle, S.; Haferkamp, H.; et al. Loading method for discrete drug depots on implant surfaces. Biomed. Eng. Biomed. Tech. 2012, 57, 1089–1092. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.-G.; Yun, I.; Chung, W.G.; Park, W.; Lee, D.H.; Park, J.-U. High-Resolution 3D Printing for Electronics. Adv. Sci. 2022, 9, e2104623. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.R.; Swennenhuis, F.; van Enckevort, W.J.; Meijer, J.A.; Vlieg, E. Creeping: An efficient way to determine the anticaking ability of additives for sodium chloride. CrystEngComm 2016, 18, 6176–6183. [Google Scholar] [CrossRef]
- van Enckevort, W.J.; Los, J.H. On the Creeping of Saturated Salt Solutions. Cryst. Growth Des. 2013, 13, 1838–1848. [Google Scholar] [CrossRef]
- Townsend, E.R.; van Enckevort, W.J.; Meijer, J.A.; Vlieg, E. Additive Enhanced Creeping of Sodium Chloride Crystals. Cryst. Growth Des. 2017, 17, 3107–3115. [Google Scholar] [CrossRef] [Green Version]
- Macielag, M.J. Chemical properties of antibacterials and their uniqueness. In Handbook of Antibiotic Discovery and Development; Thomas, J., Dougherty, M.J., Pucci, Eds.; Springer: New York, NY, USA, 2011; ISBN 978-1-4614-1400-1. [Google Scholar]
- Sigma-Aldrich. Safety Data Sheet According to Regulation (EC) No. 1907/2006. Product Name: Acetylsalicylic Acid; Product Number: A5376, CAS-No.: 50-78-2. Available online: https://www.sigmaaldrich.com/DE/en/sds/sigma/a5376 (accessed on 18 October 2022).
- Diao, Y.; Myerson, A.S.; Hatton, T.A.; Trout, B.L. Surface design for controlled crystallization: The role of surface chemistry and nanoscale pores in heterogeneous nucleation. Langmuir 2011, 27, 5324–5334. [Google Scholar] [CrossRef]
- Przybyłek, M.; Cysewski, P.; Pawelec, M.; Ziółkowska, D.; Kobierski, M. On the origin of surface imposed anisotropic growth of salicylic and acetylsalicylic acids crystals during droplet evaporation. J. Mol. Model. 2015, 21, 49. [Google Scholar] [CrossRef]
- Keith, L.H.; Walters, D.B. National Toxicology Program’s Chemical Solubility Compendium; CRC Press: Boca Raton, FL, USA, 1992; ISBN 0-87371-653-1. [Google Scholar]
- Hoath, S.D.; Jung, S.; Hutchings, I.M. A simple criterion for filament break-up in drop-on-demand inkjet printing. Phys. Fluids 2013, 25, 21701. [Google Scholar] [CrossRef] [Green Version]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Aqeel, A.B.; Mohasan, M.; Lv, P.; Yang, Y.; Duan, H. Effects of nozzle and fluid properties on the drop formation dynamics in a drop-on-demand inkjet printing. Appl. Math. Mech. Engl. Ed. 2019, 40, 1239–1254. [Google Scholar] [CrossRef]
- Gan, H.Y.; Shan, X.; Eriksson, T.; Lok, B.K.; Lam, Y.C. Reduction of droplet volume by controlling actuating waveforms in inkjet printing for micro-pattern formation. J. Micromech. Microeng. 2009, 19, 55010. [Google Scholar] [CrossRef]
- Bruner, S.; Xu, D.; Phillips, C. 54.3: Drop Landing Accuracy Improvements in Inkjet Printed OLED Displays. SID Symp. Dig. Tech. Pap. 2007, 38, 1611–1612. [Google Scholar] [CrossRef]
- Mau, R.; Oldorf, P.; Peters, R.; Seitz, H. Adjusting inkjet printhead parameters to deposit drugs into micro-sized reservoirs. Curr. Dir. Biomed. Eng. 2016, 2, 387–390. [Google Scholar] [CrossRef]
- Hsiao, H.-M.; Lin, C.-H.; Shen, Y.-K.; Chou, T.-Y.; Hsu, Y.-Y. Rhombic-Shaped Channel Stent with Enhanced Drug Capacity and Fatigue Life. Micromachines 2018, 9, 3. [Google Scholar] [CrossRef]









| Name of Drug Solution | Solvent | API | c in g/L |
|---|---|---|---|
| EtOH10ASA | EtOH | ASA | 10 |
| EtOH100ASA | EtOH | ASA | 100 |
| DMSO100ASA | DMSO | ASA | 100 |
| Medium | ρ in g/cm3 | σ in mN/m | η in mPas | Z |
|---|---|---|---|---|
| DMSO | 1.09930 ± 0.00001 | 41.52 ± 0.27 | 2.06 ± 0.06 | 17.67 |
| DMSO100ASS | 1.11314 ± 0.00002 | 42.14 ± 0.44 | 2.42 ± 0.06 | 15.24 |
| Ethanol | 0.78866 ± 0.00001 | 22.12 ± 0.15 | 1.17 ± 0.06 | 19.23 |
| ETOH100ASS | 0.82977 ± 0.00001 | 22.89 ± 0.14 | 1.28 ± 0.05 | 18.35 |
| ETOH10ASS | 0.79288 ± 0.00001 | 22.32 ± 0.10 | 1.13 ± 0.01 | 20.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mau, R.; Seitz, H. Influence of the Volatility of Solvent on the Reproducibility of Droplet Formation in Pharmaceutical Inkjet Printing. Pharmaceutics 2023, 15, 367. https://doi.org/10.3390/pharmaceutics15020367
Mau R, Seitz H. Influence of the Volatility of Solvent on the Reproducibility of Droplet Formation in Pharmaceutical Inkjet Printing. Pharmaceutics. 2023; 15(2):367. https://doi.org/10.3390/pharmaceutics15020367
Chicago/Turabian StyleMau, Robert, and Hermann Seitz. 2023. "Influence of the Volatility of Solvent on the Reproducibility of Droplet Formation in Pharmaceutical Inkjet Printing" Pharmaceutics 15, no. 2: 367. https://doi.org/10.3390/pharmaceutics15020367
APA StyleMau, R., & Seitz, H. (2023). Influence of the Volatility of Solvent on the Reproducibility of Droplet Formation in Pharmaceutical Inkjet Printing. Pharmaceutics, 15(2), 367. https://doi.org/10.3390/pharmaceutics15020367