Influence of Physicochemical Properties of Budesonide Micro-Suspensions on Their Expected Lung Delivery Using a Vibrating Mesh Nebulizer
Abstract
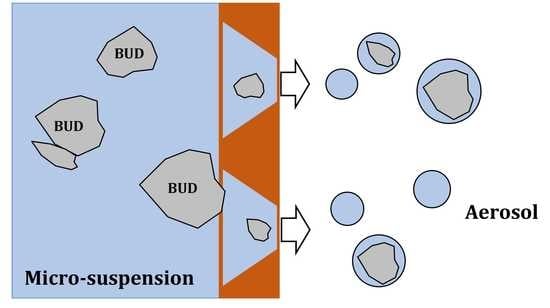
:1. Introduction
2. Materials and Methods
2.1. Micro-Suspension Stability and Electrokinetic Data
2.2. Particle Size and Morphology
2.3. Surface Tension and Rheological Properties of the Drugs
2.4. Nebulization Rate and Droplet Size Distribution
2.5. Estimation of Regional Droplet Deposition in the Respiratory Tract
2.6. The Emitted BUD Dose during Nebulization
3. Results
3.1. Microsuspension Stability and Electrokinetic Data
3.2. BUD Particle Size and Morphology
3.3. Surface Tension and Rheological Properties of the Drug
3.4. Atomization Rate and Droplet Size Distribution and Estimation of Regional Deposition of Inhaled Aerosol Droplets in the Respiratory Tract
3.5. BUD Doses
4. Discussion
- Differences in the surface tension (static and dynamic), ionic strength, and particle sedimentation rate, which can be attributed to the unequal concentrations of the adjuvants used to tune the suspension pH, isotonicity, and stability, and to extend the product shelf-life (see Table 1).
- Different size distribution of BUD crystals in the suspensions.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, J.W.; Mellon, M.; Wald, J.; Welch, M.; Cruz-Rivera, M.; Walton-Bowen, K. A multiple-dosing, placebo-controlled study of budesonide inhalation suspension given once or twice daily for treatment of persistent asthma in young children and infants. Pediatrics 1999, 103, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Agertoft, L.; Mortensen, S.; Pedersen, S. Treatment of croup with nebulised steroid (budesonide): A double blind, placebo controlled study. Arch. Dis. Child. 1993, 68, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.P.; Skoner, D.P.; Szefler, S.J.; Walton-Bowen, K.; Cruz-Rivera, M.; Smith, J.A. Once-daily budesonide inhalation suspension for the treatment of persistent asthma in infants and young children. Ann. Allergy Asthma Immunol. 1999, 83, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Mellon, M. Efficacy of budesonide inhalation suspension in infants and young children with persistent asthma. Budesonide Inhalation Suspension Study Group. J. Allergy Clin. Immunol. 1999, 104, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Szefler, S.J.; Eigen, H. Budesonide inhalation suspension: A nebulized corticosteroid for persistent asthma. J. Allergy Clin. Immunol. 2002, 109, 730–742. [Google Scholar] [CrossRef]
- FDA Approves Pulmicort Nebulized Corticosteroid (Budesonide) for Asthmatic Children. Available online: https://www.docguide.com/fda-approves-pulmicort-nebulized-corticosteroid-budesonide-asthmatic-children (accessed on 10 October 2021).
- Budesonide Inhalation Suspension. Available online: https://www.drugs.com/pro/budesonide-inhalation-suspension.html (accessed on 6 December 2022).
- Dhand, R. Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respir. Care 2002, 47, 1406–1418. [Google Scholar]
- Martin, A.R.; Finlay, W.H. Nebulizers for drug delivery to the lungs. Expert Opin. Drug Deliv. 2015, 12, 889–900. [Google Scholar] [CrossRef]
- Pritchard, J.N.; Hatley, R.H.; Denyer, J.; Hollen, D.V. Mesh nebulizers have become the first choice for new nebulized pharmaceutical drug developments. Ther. Deliv. 2018, 9, 121–136. [Google Scholar] [CrossRef] [Green Version]
- Ari, A.; de Andrade, A.D.; Sheard, M.; Al Hamad, B.; Fink, J.B. Performance comparisons of jet and mesh nebulizers using different interfaces in simulated spontaneously breathing adults and children. J. Aerosol. Med. Pulm. Drug Deliv. 2015, 28, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zhang, R.; Beng, H.; Deng, L.; Ke, Q.; Tan, W. Effects of flow pattern, device and formulation on particle size distribution of nebulized aerosol. Int. J. Pharm. 2019, 560, 35–46. [Google Scholar] [CrossRef]
- Murayama, N.; Asai, K.; Murayama, K.; Doi, S.; Kameda, M. Dry powder and budesonide inhalation suspension deposition rates in asthmatic airway-obstruction regions. J. Drug Deliv. 2019, 2019, 3921426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schueepp, K.G.; Devadason, S.G.; Roller, C.; Minocchieri, S.; Moeller, A.; Hamacher, J.; Wildhaber, J.H. Aerosol delivery of nebulised budesonide in young children with asthma. Respir. Med. 2009, 103, 1738–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schueepp, K.G.; Devadason, S.; Roller, C.; Wildhaber, J.H. A complementary combination of delivery device and drug formulation for inhalation therapy in preschool children. Swiss Med. Wkly 2004, 134, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, J.; Motomura, C.; Ikei, J.; Ide, K.; Kando, N.; Goto, M.; Taba, N.; Hayashi, D.; Murakami, Y.; Moriyasu, Y.; et al. Efficacy and safety of budesonide inhalation suspension nebulization by mesh nebulizer in Japanese infants and young children with bronchial asthma in 12-week, randomised, open study. Arerugi 2008, 57, 1034–1042. [Google Scholar]
- Fink, J.B.; Simmons, R. Nebulization of steroid suspension: An in vitro evaluation of the Aeroneb® Go and Pari LC Plus™ nebulizers. Chest 2004, 126, S816–S817. [Google Scholar] [CrossRef]
- Luangkhot, N.; Jauernig, J.; Balcke, A.; Lintz, F.C.; Liening-Ewert, R.; Stangl, R.; Keller, M. Characterisation of salbutamol solution compared to budesonide suspensions consisting of submicron and micrometer particles in the PARI LC STAR® and a new PARI electronic Nebuliser (e-Flow). In Drug Delivery to the Lungs XI; The Aerosol Society: London, UK, 2000; pp. 14–17. [Google Scholar]
- Slator, L.; Cooper-Rayner, N.; Hardaker, L.E.; Hollen, D.V.; Pritchard, J.N. Delivery of a budesonide suspension formulation from mesh vs jet nebulizers under simulated pediatric and adult breathing patterns. J. Aerosol. Med. Pulm. Drug Deliv. 2018, 31, A13–A14. [Google Scholar]
- Slator, L.; Quadrelli, F.; Hardaker, L.E.A.; Hatley, R.H.M. Aerosol particle size characterization of several common respiratory formulations from a novel handheld mesh nebulizer. In Respiratory Drug Delivery Europe 2017; Dalby, R.N., Peart, J., Suman, J.D., Young, P.M., Traini, D., Eds.; Virginia Commonwealth University: Richmond, VA, USA, 2017; pp. 289–292. [Google Scholar]
- Sosnowski, T.R.; Rapiejko, P.; Sova, J.; Dobrowolska, K. Impact of physicochemical properties of nasal spray products on drug deposition and transport in the pediatric nasal cavity model. Int. J. Pharm. 2020, 574, 118911. [Google Scholar] [CrossRef]
- Twister Mesh NE-105—Operation Manual (in Polish). Available online: https://www.intecmedical.pl/inhalator-membranowy-siateczkowy-intec-twister-mesh.html (accessed on 6 December 2022).
- Aura Portable Nebulizer—Operation Manual. Available online: https://www.aura-medical.com/wp-content/uploads/2019/05/AuraMedical_A6_32_portaneb_Notforprinting.pdf (accessed on 6 December 2022).
- Finlay, W.H. The Mechanics of Inhaled Pharmaceutical Aerosols. An Introduction; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Ziegler, J.; Wachtel, H. Comparison of cascade impaction and laser diffraction for particle size distribution measurements. J. Aerosol. Med. 2005, 18, 311–324. [Google Scholar] [CrossRef]
- Mao, L.; Wilcox, D.; Kippax, P. Laser diffraction particle size analysis: A powerful tool for rapidly screening nebulizer formulations. Drug Deliv. Techn. 2010, 10, 64–67. [Google Scholar]
- Bauer, A.; McGlynn, P.; Bovet, L.L.; Mims, P.L.; Curry, L.A.; Hanrahan, J.P. Output and aerosol properties of 5 nebulizer/compressor systems with arformoterol inhalation solution. Respir. Care 2009, 54, 1342. [Google Scholar]
- Pham, S.; Ferguson, G.T.; Kerwin, E.; Goodin, T.; Wheeler, A.; Bauer, A. In vitro characterization of the eFlow closed system nebulizer with glycopyrrolate inhalation solution. J. Aerosol Med. Pulm. Drug Del. 2018, 31, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Schuschnig, U.; Heine, B.; Knoch, M. How cold is cold enough? Refrigeration of the Next-Generation Impactor to prevent aerosol undersizing. J. Aerosol Med. Pulm. Drug Del. 2022, 35, 25–31. [Google Scholar] [CrossRef]
- MPPD: Multiple-Path Particle Dosimetry Model. Applied Research Associates Inc. Available online: https://www.ara.com/mppd/ (accessed on 6 December 2022).
- Yeh, H.C.; Schum, G.M. Models of human lung airways and their application to inhaled particle deposition. Bull. Math. Biol. 1980, 42, 461–480. [Google Scholar] [CrossRef]
- ACQUITY UPLC Photodiode Array and eλ Photodiode Array Detector, Operator’s Overview and Maintenance Guide. Available online: https://www.waters.com/webassets/cms/support/docs/715002209ra.pdf (accessed on 6 December 2022).
- Chen, C.M.; Chang, C.H.; Chao, C.H.; Wang, M.H.; Yeh, T.F. Biophysical and chemical stability of surfactant/budesonide and the pulmonary distribution following intra-tracheal administration. Drug Deliv. 2019, 26, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Ghazanfari, T.; Elhissi, A.M.A.; Ding, Z.; Taylor, K.M.G. The influence of fluid physicochemical properties on vibrating-mesh nebulization. Int. J. Pharmacol. 2007, 339, 103–111. [Google Scholar] [CrossRef]
- Hardaker, L.E.; Hatley, R.H. In vitro characterization of the I-neb Adaptive Aerosol Delivery (AAD) system. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, S11–S20. [Google Scholar] [CrossRef] [Green Version]
- Beck-Broichsitter, M.; Knuedeler, M.-C.; Seeger, W.; Schehl, T. Controlling the droplet size of formulations nebulized by vibrating-membrane technology. Eur. J. Pharm. Biopharm. 2014, 87, 524–529. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Gradoń, L. The effect of dynamic surface tension on the output of pneumatic nebulization. J. Aerosol. Sci. 2001, 32S1, S795–S796. [Google Scholar] [CrossRef]
- Barnes, P.J.; Pedersen, S.; Busse, W.W. Efficacy and safety of inhaled corticosteroids. New developments. Am. J. Respir. Crit. Care Med. 1998, 157, S1–S53. [Google Scholar] [CrossRef]
- Arzhavitina, A.; Steckel, H. Surface active drugs significantly alter the drug output rate from medical nebulizers. Int. J. Pharm. 2010, 384, 128–136. [Google Scholar] [CrossRef]
- McCallion, O.N.; Taylor, K.M.; Thomas, M.; Taylor, A.J. Nebulization of fluids of different physicochemical properties with air-jet and ultrasonic nebulizers. Pharm. Res. 1995, 12, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Broniarz-Press, L.; Sosnowski, T.R.; Matuszak, M.; Ochowiak, M.; Jabłczyńska, K. The effect of shear and extensional viscosities on atomization of Newtonian and non-Newtonian fluids in ultrasonic inhaler. Int. J. Pharm. 2015, 485, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.C.; Peters, J.I.; Williams, R.O., 3rd. Influence of particle size on regional lung deposition—What evidence is there? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pirożyński, M.; Sosnowski, T.R. Inhalation devices: From basic science to practical use, innovative vs generic products. Expert Opin. Drug Deliv. 2016, 13, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Respiratory Therapy Equipment: Nebulizing Systems and Their Components; European Committee for Standardization; CEN: EN 13544-1:2007+ A1:2009; British Standard Institute: London, UK, 2010.
- Anaesthetic and Respiratory Equipment—Nebulizing Systems and Components. ISO 27427:2013. Available online: https://www.iso.org/obp/ui#iso:std:iso:27427:ed-3:v1:en (accessed on 6 December 2022).
- Sahib, M.N.; Darwis, Y.; Peh, K.K.; Abdulameer, S.A.; Tan, Y.T. Rehydrated sterically stabilized phospholipid nanomicelles of budesonide for nebulization: Physicochemical characterization and in vitro, in vivo evaluations. Int. J. Nanomed. 2011, 6, 2351–2366. [Google Scholar] [CrossRef] [Green Version]
- Sosnowski, T.R. Critical assessment of the quantitative criteria used in the comparison of nebulizers. EC Pulmonol. Respir. Med. 2019, 8, 656–662. [Google Scholar]
- Kovalchuk, N.; Starov, V.; Holdich, R. Effect of aggregation on viscosity of colloidal suspension. Colloid J. 2010, 72, 647–652. [Google Scholar] [CrossRef] [Green Version]
- O’Riordan, T.G. Formulations and nebulizer performance. Respir Care 2002, 47, 1305–1313. [Google Scholar]
- Lass, J.S.; Sant, A.; Knoch, M. New advances in aerosolised drug delivery: Vibrating membrane nebuliser technology. Expert Opin. Drug Deliv. 2006, 3, 693–702. [Google Scholar] [CrossRef]
- Dolovich, M.B.; Dhand, R. Aerosol drug delivery: Developments in device design and clinical use. Lancet 2011, 377, 1032–1045. [Google Scholar] [CrossRef]
- Choi, K.H.; Moon, S.H.; Park, S.K.; Cho, G.; Nam, K.C.; Park, B.J. Fabrication and characterization of medical mesh-nebulizer for aerosol drug delivery. Appl. Sci. 2018, 8, 604. [Google Scholar] [CrossRef] [Green Version]
- Hatley, R.; Hardacker, L.; Zarins-Tutt, J.; Quadrelli, F.; Hogan, B.; MacLoughlin, R.; Pritchard, J.N. Investigation of optical density for the characterization of nebulizer meshes. In Respiratory Drug Delivery 2016; Virginia Commonwealth University: Richmond, VA, USA, 2016; Volume 3, pp. 489–492. [Google Scholar]
- Dobrowolska, K.; Sosnowski, T.R. Evolution of droplet size distribution in selected nebulizers. Physicochem. Probl. Min. Proc. 2020, 56, 32–40. [Google Scholar] [CrossRef]
- Mazela, J.; Chmura, K.; Kulza, M.; Henderson, C.; Gregory, T.J.; Moskal, A.; Sosnowski, T.R.; Florek, E.; Kramer, L.; Keszler, M. Aerosolized albuterol sulfate delivery under neonatal ventilatory conditions: In vitro evaluation of a novel ventilator circuit patient interface connector. J. Aerosol Med. Pulm. Drug Del. 2014, 27, 58–65. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Janeczek, K.; Grzywna, K.; Emeryk, A. Mass and volume balances of nebulization processes for the determination of the expected dose of liquid medicines delivered by inhalation. Chem. Proc. Eng. 2021, 47, 253–261. [Google Scholar]
- Shrewsbury, S.B.; Bosco, A.P.; Uster, P.S. Pharmacokinetics of a novel submicron budesonide dispersion for nebulized delivery in asthma. Int. J. Pharm. 2009, 365, 12–17. [Google Scholar] [CrossRef]
- Britland, S.; Finter, W.; Chrystyn, H.; Eagland, D.; Abdelrahim, M.E. Droplet aerodynamics, cellular uptake, and efficacy of a nebulizable corticosteroid nanosuspension are superior to a micronized dosage form. Biotechnol. Prog. 2012, 28, 1152–1159. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J. Preparation of budesonide nanosuspensions for pulmonary delivery: Characterization, in vitro release and in vivo lung distribution studies. Artif. Cells Nanomed. Biotechnol. 2016, 44, 285–289. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Dobrowolska, K. In vitro interactions of nebulized budesonide inhalation suspension with the lung surfactant: The importance of drug formulation. In Respiratory Drug Delivery 2020; Virginia Commonwealth University: Richmond, VA, USA, 2020; pp. 737–742. [Google Scholar]
- Lu, D.; Lee, S.L.; Lionberger, R.A.; Choi, S.; Adams, W.; Caramenico, H.N.; Chowdhury, B.A.; Conner, D.P.; Katial, R.; Limb, S.; et al. International guidelines for bioequivalence of locally acting orally inhaled drug products: Similarities and differences. AAPS J. 2015, 17, 546–557. [Google Scholar] [CrossRef] [Green Version]
- CHMP. Guideline on the Requirements for Clinical Documentation for Orally Inhaled Products (OIP) Including the Requirements for Demonstration of Therapeutic Equivalence between Two Inhaled Products for Use in the Treatment of Asthma and Chronic Obstructive Pulmonary Disease (COPD) in Adults and for Use in the Treatment of Asthma in Children and Adolescents; European Medicine Agency: London, UK, 2009. [Google Scholar]
- CHMP. Guideline on the Pharmaceutical Quality of Inhalation and Nasal Products; European Medicine Agency: London, UK, 2006. [Google Scholar]
- Szeitz, A.; Manji, J.; Riggs, K.W.; Thamboo, K.; Javer, A.R. Validated assay for the simultaneous determination of cortisol and budesonide in human plasma using ultra high performance liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 90, 198–206. [Google Scholar] [CrossRef]
- Rao, B.T.; Mantha, S.; Vaidyanathan, G. Quantification of Budesonide Using UPLC and Xevo TQ-S. Application Note, Waters Corp. Available online: https://www.waters.com/content/dam/waters/en/app-notes/2012/720004198/720004198-es.pdf (accessed on 15 February 2023).











| BUD Suspension | A | B | C | D |
|---|---|---|---|---|
| Active ingredient | budesonide (0.5 mg/mL) | budesonide (0.5 mg/mL) | budesonide (0.5 mg/mL) | budesonide (0.5 mg/mL) |
| Additives 1: | ||||
| EDTA Na2 | + | dihydrate | + | + |
| NaCl | + | + | + | + |
| polysorbate 80 | + | + | + | + |
| citric acid | + | monohydrate | + | + |
| solvent | purified water | water for injection | water for injection | water for injection |
| Type of Experiment | Method/Equipment |
|---|---|
| Suspension stability | UV spectrometry: GENESYS 10S UV-Vis (Thermo Scientific, Waltham, MA, USA) |
| Electrokinetic properties (ζ-potential, conductivity, pH) | Zetasizer Nano ZS (Malvern, Worcestershire, UK), conductivity and pH-meter (Elmetron, Zabrze, Poland) |
| Dynamic and static surface tension | Pendant drop tensiometry: PAT-1M (Sinterface, Berlin, Germany) |
| Rheological characteristics | Plate–plate rheometry: MCR 102 (Anton Paar, Graz, Austria) |
| Nebulizer mass output | Gravimetry: analytical balance (Radwag, Radom, Poland) |
| BUD particle morphology and size | Scanning electron microscopy: TM-1000 (Hitachi, Tokyo, Japan) |
| BUD particle size distribution | Laser diffraction in liquid: LS 13 320 XR (Beckman Coulter, Brea, CA, USA) |
| BUD mass-delivered from the nebulizer | Ultra-high-performance liquid chromatography (UPLC) (Waters, Milford, CT, USA) |
| Aerosol droplet size distribution | Laser diffraction in air: Spraytec (Malvern, Worcestershire, UK) |
| BUD Suspension | BUD Concentration in the Sample (mg/mL) | pH | Conductivity (mS/cm) | Zeta Potential (mV) | ||
|---|---|---|---|---|---|---|
| κ | SD | ζ | SD | |||
| A | 0.0313 * | ~7 | 3.85 | 0.15 | −4.32 | 0.43 |
| B | 0.0625 | ~7 | 3.98 | 0.17 | −3.14 | 0.14 |
| C | 0.0625 | ~7 | 2.66 | 0.06 | −6.26 | 0.31 |
| D | 0.0625 | ~7 | 4.84 | 0.29 | −4.70 | 0.77 |
| BUD Suspension | Quasi-Equilibrium Surface Tension σ, mN/m | SD mN/m |
|---|---|---|
| A | 41.9 | 0.6 |
| B | 42.6 | 1.1 |
| C | 40.2 | 0.1 |
| D | 40.3 | 0.3 |
| BUD Suspension | Output Rate, g/min | SD, g/min |
|---|---|---|
| A | 0.396 | 0.027 |
| B | 0.336 | 0.021 |
| C | 0.424 | 0.023 |
| D | 0.344 | 0.011 |
| BUD Suspension | Deposition Efficiency | |||
|---|---|---|---|---|
| Oropharynx (Mouth and Throat) | Trachea + Bronchi | Pulmonary Region | Total in the Lower Airways | |
| A | 29.4% | 25.0% | 20.0% | 45.0% |
| B | 29.9% | 25.1% | 19.8% | 44.9% |
| C | 27.0% | 24.9% | 21.2% | 46.1% |
| D | 29.9% | 25.1% | 19.8% | 44.9% |
| BUD Suspension | Actual BUD Dose, ADBUD, μg Average ± SD | Nebulized BUD Dose, NDBUD, μg Average ± SD | Average Residual BUD Mass in Relation to Actual Dose, RFBUD, % |
|---|---|---|---|
| A | 1018.2 ± 20.5 | 875.0 ± 60.6 | 14.1 |
| B | 1023.5 ± 14.2 | 777.4 ± 48.6 | 24.0 |
| C | 1024.2 ± 27.0 | 875.7 ± 38.5 | 14.5 |
| D | 1025.6 ± 20.2 | 890.5 ± 19.9 | 13.2 |
| A | B | C | |
|---|---|---|---|
| B | <0.001 | - | <0.001 |
| C | 1.00 | <0.001 | - |
| D | 0.91 | <0.001 | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrowolska, K.; Emeryk, A.; Janeczek, K.; Krzosa, R.; Pirożyński, M.; Sosnowski, T.R. Influence of Physicochemical Properties of Budesonide Micro-Suspensions on Their Expected Lung Delivery Using a Vibrating Mesh Nebulizer. Pharmaceutics 2023, 15, 752. https://doi.org/10.3390/pharmaceutics15030752
Dobrowolska K, Emeryk A, Janeczek K, Krzosa R, Pirożyński M, Sosnowski TR. Influence of Physicochemical Properties of Budesonide Micro-Suspensions on Their Expected Lung Delivery Using a Vibrating Mesh Nebulizer. Pharmaceutics. 2023; 15(3):752. https://doi.org/10.3390/pharmaceutics15030752
Chicago/Turabian StyleDobrowolska, Katarzyna, Andrzej Emeryk, Kamil Janeczek, Radosław Krzosa, Michał Pirożyński, and Tomasz R. Sosnowski. 2023. "Influence of Physicochemical Properties of Budesonide Micro-Suspensions on Their Expected Lung Delivery Using a Vibrating Mesh Nebulizer" Pharmaceutics 15, no. 3: 752. https://doi.org/10.3390/pharmaceutics15030752
APA StyleDobrowolska, K., Emeryk, A., Janeczek, K., Krzosa, R., Pirożyński, M., & Sosnowski, T. R. (2023). Influence of Physicochemical Properties of Budesonide Micro-Suspensions on Their Expected Lung Delivery Using a Vibrating Mesh Nebulizer. Pharmaceutics, 15(3), 752. https://doi.org/10.3390/pharmaceutics15030752