Local Drug Delivery Strategies towards Wound Healing
Abstract
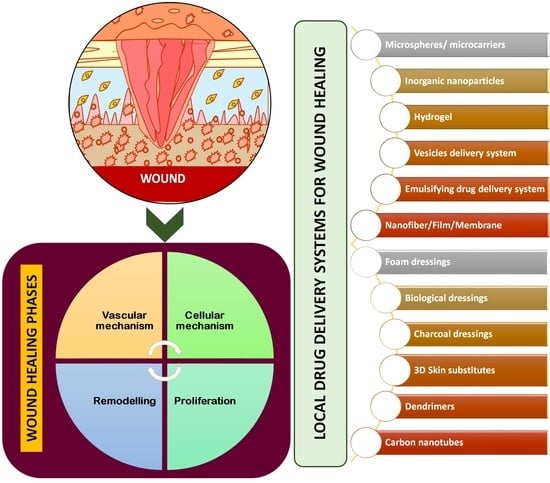
:1. Introduction
2. Physiology of Wound Healing Process
2.1. Haemostasis and Coagulation: Vascular Mechanism
2.2. Inflammation: Cellular Mechanism
2.3. Proliferation
2.3.1. Epithelialisation
2.3.2. Angiogenesis
2.3.3. Granulation Tissue Formation
2.4. Remodelling Phase
3. Wound Healing Strategies
3.1. Cellular Activity Initiators
3.2. Collagen Synthesis Activators
3.3. Angiogenesis Activators
3.4. Cytokine and Growth Factor Activators
3.5. Antimicrobials
3.6. Stem Cell-Based Therapy
3.7. Herbal Alternatives Acting as Activators for Wound Healing Factors
4. Localised Delivery Systems for Wound Healing
4.1. Microspheres/Microcarriers
4.2. Inorganic Nanoparticles
4.3. Hydrogel
4.4. Vesicles Delivery System
4.4.1. Conventional Liposomes in Wound Healing
4.4.2. Ultra-Deformable Liposomes or Transferosomes in Wound Healing
4.4.3. Ethosomes and Phytosomes in Wound Healing
4.5. Emulsifying Drug Delivery System
4.6. Nanofiber/Film/Membrane
4.7. Foam Dressings
4.8. Biological Dressings
4.9. Charcoal Dressings
4.10. Three-Dimensional Skin Substitutes
4.11. Dendrimers
4.12. Carbon Nanotubes
4.13. Microneedle Drug Delivery Systems
5. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.W.; Xian, C.J. Adipose-derived stem cells for wound healing. J. Cell. Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef] [PubMed]
- Ryall, C.; Duarah, S.; Chen, S.; Yu, H.; Wen, J. Advancements in Skin Delivery of Natural Bioactive Products for Wound Management: A Brief Review of Two Decades. Pharmaceutics 2022, 14, 1072. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhang, K.; Jiao, X.; Cheng, Y.; Zhang, Y.; Zhang, P.; Zhang, X.; Wen, Y. A controllable local drug delivery system based on porous fibers for synergistic treatment of melanoma and promoting wound healing. Biomater. Sci. 2019, 7, 5084–5096. [Google Scholar] [CrossRef] [PubMed]
- Elviri, L.; Bianchera, A.; Bergonzi, C.; Bettini, R. Controlled local drug delivery strategies from chitosan hydrogels for wound healing. Expert Opin. Drug Deliv. 2017, 14, 897–908. [Google Scholar] [CrossRef]
- Gorain, B.; Pandey, M.; Leng, N.H.; Yan, C.W.; Nie, K.W.; Kaur, S.J.; Marshall, V.; Sisinthy, S.P.; Panneerselvam, J.; Molugulu, N.; et al. Advanced drug delivery systems containing herbal components for wound healing. Int. J. Pharm. 2022, 617, 121617. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Aleshkin, A.; Bochkareva, S.; Modin, E.; Mashaqi, B.; Boyle, E.C.; Boethig, D.; Rubalsky, M.; et al. Fibrin glue as a local drug-delivery system for bacteriophage PA5. Sci. Rep. 2019, 9, 2091. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.J.; Bae, S.K.; Jung, Y.S.; Kim, J.C.; Kim, J.S.; Park, S.K.; Suh, J.S.; Yi, S.J.; Ahn, S.H.; Lim, J.O. Enhanced wound healing using a 3D printed VEGF-mimicking peptide incorporated hydrogel patch in a pig model. Biomed. Mater. 2021, 16, 045013. [Google Scholar] [CrossRef] [PubMed]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid. Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef]
- Fatehi, P.; Abbasi, M. Medicinal plants used in wound dressings made of electrospun nanofibers. J. Tissue Eng. Regen. Med. 2020, 14, 1527–1548. [Google Scholar] [CrossRef]
- Wang, Y.; Malcolm, D.W.; Benoit, D.S.W. Controlled and sustained delivery of siRNA/NPs from hydrogels expedites bone fracture healing. Biomaterials 2017, 139, 127–138. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Wang, W.; Lu, K.J.; Yu, C.H.; Huang, Q.L.; Du, Y.Z. Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotechnol. 2019, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, e2100477. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, D.P. Photobiomodulation in promoting wound healing: A review. Regen. Med. 2016, 11, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, K.K.; Vandermeulen, G.; Préat, V. PLGA based drug delivery systems: Promising carriers for wound healing activity. Wound Repair Regen. 2016, 24, 223–236. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and physiology of wound healing. Clin. Plast. Surg. 2012, 39, 85–97. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Kaiser, P.; Wächter, J.; Windbergs, M. Therapy of infected wounds: Overcoming clinical challenges by advanced drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 1545–1567. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Broughton, G.; Janis, J.E.; Attinger, C.E. Wound healing: An overview. PlastReconstr. Surg. 2006, 117 (Suppl. 7), 1e-S–32e-S. [Google Scholar] [CrossRef] [Green Version]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Ud-Din, S.; Bayat, A. Non-animal models of wound healing in cutaneous repair: In silico, in vitro, ex vivo, and in vivo models of wounds and scars in human skin. Wound Repair Regen. 2017, 25, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Masoumpour, M.B.; Nowroozzadeh, M.H.; Razeghinejad, M.R. Current and Future Techniques in Wound Healing Modulation after Glaucoma Filtering Surgeries. Open Ophthalmol. J. 2016, 10, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Cabourne, E.; Clarke, J.C.; Schlottmann, P.G.; Evans, J.R. Mitomycin C versus 5-Fluorouracil for wound healing in glaucoma surgery. Cochrane Database Syst. Rev. 2015, 2015, CD006259. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, S.E.; MoridiFarimani, M.; Soroury, S.; Ebrahimi, S.N.; Jabbarzadeh, E. Hypermongone C Accelerates Wound Healing through the Modulation of Inflammatory Factors and Promotion of Fibroblast Migration. Molecules 2019, 24, 2022. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, M.; Kakpenova, A.; Simon, J.C.; Franz, S. Modulation of macrophage functions by ECM-inspired wound dressings—A promising therapeutic approach for chronic wounds. Biol. Chem. 2021, 402, 1289–1307. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Wojcik, M.; Palka, K.; Przekora, A. Highly Porous and Superabsorbent Biomaterial Made of Marine-Derived Polysaccharides and Ascorbic Acid as an Optimal Dressing for Exuding Wound Management. Materials 2021, 14, 1211. [Google Scholar] [CrossRef]
- Westby, M.J.; Dumville, J.C.; Soares, M.O.; Stubbs, N.; Norman, G. Dressings and topical agents for treating pressure ulcers. Cochrane Database Syst. Rev. 2017, 6, CD011947. [Google Scholar] [CrossRef]
- Berger, M.M.; Binz, P.A.; Roux, C.; Charrière, M.; Scaletta, C.; Raffoul, W.; Applegate, L.A.; Pantet, O. Exudative glutamine losses contribute to high needs after burn injury. J. Parenter. Enter. Nutr. 2022, 46, 782–788. [Google Scholar] [CrossRef]
- Guan, Y.; Niu, H.; Liu, Z.; Dang, Y.; Shen, J.; Zayed, M.; Ma, L.; Guan, J. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci. Adv. 2021, 7, eabj0153. [Google Scholar] [CrossRef] [PubMed]
- Bossi, F.; Tripodo, C.; Rizzi, L.; Bulla, R.; Agostinis, C.; Guarnotta, C.; Munaut, C.; Baldassarre, G.; Papa, G.; Zorzet, S.; et al. C1q as a unique player in angiogenesis with therapeutic implication in wound healing. Proc. Natl. Acad. Sci. USA 2014, 111, 4209–4214. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Guo, X.; Gao, D. R-responsive MXene nanobelts for wound healing. NPG Asia Mater. 2021, 13, 24. [Google Scholar] [CrossRef]
- Vijayan, V.; Sreekumar, S.; Singh, F.; Govindarajan, D.; Lakra, R.; Korrapati, P.-S.; Kiran, M.S. Praseodymium-Cobaltite-Reinforced Collagen as Biomimetic Scaffolds for Angiogenesis and Stem Cell Differentiation for Cutaneous Wound Healing. ACS Appl. Bio Mater. 2019, 2, 3458–3472. [Google Scholar] [CrossRef] [PubMed]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Yan, Z.; Xiao, S.; Xia, Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res. Ther. 2020, 11, 232. [Google Scholar] [CrossRef]
- Heinrich, P.-C.; Behrmann, I.; Haan, S.; Hermanns, H.-M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 15, 374. [Google Scholar] [CrossRef]
- Mi, F.L.; Wu, Y.B.; Shyu, S.S.; Schoung, J.Y.; Huang, Y.B.; Tsai, Y.H.; Hao, J.Y. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J. Biomed. Mater. Res. 2002, 59, 438–449. [Google Scholar] [CrossRef]
- Tamahkar, E.; Özkahraman, B.; Süloğlu, A.K.; İdil, N.; Perçin, I. A novel multilayer hydrogel wound dressing for antibiotic release. J. Drug Deliv. Sci. Technol. 2020, 58, 101536. [Google Scholar] [CrossRef]
- Sabitha, M.; Rajiv, S. Preparation and characterization of ampicillin-incorporated electrospun polyurethane scaffolds for wound healing and infection control. Polym. Eng. Sci. 2015, 55, 541–548. [Google Scholar] [CrossRef]
- Ye, S.; Jiang, L.; Wu, J.; Su, C.; Huang, C.; Liu, X.; Shao, W. Flexible amoxicillin-grafted bacterial cellulose sponges for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2018, 10, 5862–5870. [Google Scholar] [CrossRef]
- Basha, M.; AbouSamra, M.M.; Awad, G.A.; Mansy, S.S. A potential antibacterial wound dressing of cefadroxil chitosan nanoparticles in situ gel: Fabrication, in vitro optimization and in vivo evaluation. Int. J. Pharm. 2018, 544, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Nikdel, M.; Rajabinejad, H.; Yaghoubi, H.; Mikaeiliagah, E.; Cella, M.A.; Sadeghianmaryan, A.; Ahmadi, A. Fabrication of cellulosic nonwoven material coated with polyvinyl alcohol and zinc oxide/mesoporous silica nanoparticles for wound dressing purposes with cephalexin delivery. ECS J. Solid State Sci. Technol. 2021, 10, 057003. [Google Scholar] [CrossRef]
- Rădulescu, M.; Holban, A.-M.; Mogoantă, L.; Bălşeanu, T.-A.; Mogos-anu, G.-D.; Savu, D.; Popescu, R.C.; Fufă, O.; Grumezescu, A.M.; Bezirtzoglou, E.; et al. Fabrication, Characterization, and Evaluation of Bionanocomposites Based on Natural Polymers and Antibiotics for Wound Healing Applications. Molecules 2016, 21, 761. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Boni, B.O.O.; Ahmed, A.A.Q.; Zheng, R.; Shi, Z.; Ullah, M.W.; Lamboni, L.; Yang, G. In Situ Synthesized Porous Bacterial Cellulose/Poly (vinyl alcohol)-Based Silk Sericin and Azithromycin Release System for Treating Chronic Wound Biofilm. Macromol. Biosci. 2022, 1, 2200201. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, F.; Ayan, S.; Duygulu, N.; Yilmazer, Y.; Karavelioglu, Z.; Vehapi, M.; ÇakırKoç, R.; Sengor, M.; Yılmazer, H.; Ozcimen, D.; et al. Selenium and clarithromycin loaded PLA-GO composite wound dressings by electrospinning method. Int. J. Polym. Mater. Polym. Biomater. 2022, 13, 71. [Google Scholar] [CrossRef]
- de Souza, R.F.B.; de Souza, F.C.B.; Moraes, Â.M. Polysaccharide-based membranes loaded with erythromycin for application as wound dressings. Appl. Polym. Sci. 2016, 10, 133. [Google Scholar] [CrossRef]
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.J. Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng. C 2017, 1, 77. [Google Scholar] [CrossRef]
- Khampieng, T.; Wnek, G.-E.; Supaphol, P. Electrospun DOXY-h loaded-poly(acrylic acid) nanofiber mats:In vitro drug release and antibacterial properties investigation. J. Biomater. Sci. Polym. Ed. 2014, 25, 1292–1305. [Google Scholar] [CrossRef]
- Akota, I.; Alvsaker, B.; Bjørnland, T. The effect of locally applied gauze drain impregnated with chlortetracycline ointment in mandibular third-molar surgery. Acta Odontol. Scand. 1998, 56, 25–29. [Google Scholar] [CrossRef]
- Abbott, P.V.; Hume, W.R.; Pearman, J.W. Antibiotics and endodontics. Aust. Dental. J. 1990, 35, 50–60. [Google Scholar] [CrossRef]
- Michalska-Sionkowska, M.; Kaczmarek, B.; Walczak, M.; Sionkowska, A. Antimicrobial activity of new materials based on the blends of collagen/chitosan/hyaluronic acid with gentamicin sulfate addition. Mater. Sci. Eng. C 2018, 1, 86. [Google Scholar] [CrossRef]
- Anjum, A.; Sim, C.H.; Ng, S.F. Hydrogels containing antibiofilm and antimicrobial agents beneficial for biofilm-associated wound infection: Formulation characterizations and In vitro study. AAPS PharmSciTech 2018, 19, 1219–1230. [Google Scholar] [CrossRef]
- Ahire, J.J.; Robertson, D.D.; van Reenen, A.J.; Dicks, L.M.T. Polyethylene oxide (PEO)-hyaluronic acid (HA) nanofibers with kanamycin inhibits the growth of Listeria monocytogenes. Biomed. Pharmacother. 2017, 86, 143–148. [Google Scholar] [CrossRef]
- Nitanan, T.; Akkaramongkolporn, P.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Neomycin-loaded poly (styrene sulfonic acid-co-maleic acid) (PSSA-MA)/polyvinyl alcohol (PVA) ion exchange nanofibers for wound dressing materials. Int. J. Pharm. 2013, 1, 448. [Google Scholar] [CrossRef]
- Denkbaş, E.U.R.B.; Öztürk, E.; Özdem&unknownr, N.; Agalar, C. Norfloxacin-loaded chitosan sponges as wound dressing material. J. Biomater. Appl. 2004, 18, 291–303. [Google Scholar] [CrossRef]
- Contardi, M.; Heredia-Guerrero, J.A.; Perotto, G.; Valentini, P.; Pompa, P.P.; Spanò, R.; Goldonic, L.; Bertorelli, R.; Athanassiou, A.; Bayera, I.S. Transparent ciprofloxacin-povidone antibiotic films and nanofiber mats as potential skin and wound care dressings. Eur. J. Pharm. Sci. 2017, 104, 133–144. [Google Scholar] [CrossRef]
- Li, H.; Williams, G.R.; Wu, J.; Wang, H.; Sun, X.; Zhu, L.M. Poly (N-isopropylacrylamide)/poly (l-lactic acid-co-ɛ-caprolactone) fibers loaded with ciprofloxacin as wound dressing materials. Mater. Sci. Eng. C 2017, 1, 79. [Google Scholar] [CrossRef]
- Pamfil, D.; Vasile, C.; Tarţău, L.; Vereştiuc, L.; Poiată, A. pH-Responsive 2-hydroxyethyl methacrylate/citraconic anhydride–modified collagen hydrogels as ciprofloxacin carriers for wound dressings. J. Bioact. Compat. Polym. 2017, 32, 355–381. [Google Scholar] [CrossRef]
- Pásztor, N.; Rédai, E.; Szabó, Z.I.; Sipos, E. Preparation and Characterization of Levofloxacin-Loaded Nanofibers as Potential Wound Dressings. Acta Med. Marisiensis 2017, 1, 63. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Dhiman, A. Designing bio-mimetic moxifloxacin loaded hydrogel wound dressing to improve antioxidant and pharmacology properties. RSC Adv. 2015, 5, 44666–44678. [Google Scholar] [CrossRef]
- Kurczewska, J.; Pecyna, P.; Ratajczak, M.; Gajęcka, M.; Schroeder, G. Halloysite nanotubes as carriers of vancomycin in alginate-based wound dressing. Saudi Pharm. J. 2017, 1, 25. [Google Scholar] [CrossRef]
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Darban, S.A.; Bazzaz, B.S.F.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing. Int. J. Biol. Macromol. 2020, 162, 645–656. [Google Scholar] [CrossRef]
- Rolston, K.V.I.; Dholakia, N.; Ho, D.H.; LeBlanc, B.; Dvorak, T.; Streeter, H. In-vitro activity of ramoplanin (a novel lipoglycopeptide), vancomycin, and teicoplanin against gram-positive clinical isolates from cancer patients. J. Antimicrob. Chemother. 1996, 38, 265–269. [Google Scholar] [CrossRef]
- Habash, M.B.; Park, A.J.; Vis, E.C.; Harris, R.J.; Khursigara, C.M. Synergy of silver nanoparticles and aztreonam against Pseudomonas aeruginosa PAO1 biofilms. Antimicrob. Agents Chemother. 2014, 58, 5818–5830. [Google Scholar] [CrossRef]
- Jones, R.N. Critical assessment of the newer non-quinolone oral antimicrobial agents. Antimicrob. Newsl. 1989, 6, 53–60. [Google Scholar] [CrossRef]
- Bauernfeind, A.; Schweighart, S.; Chong, Y. Extended broad spectrum β-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection 1989, 17, 316–321. [Google Scholar] [CrossRef]
- Teaima, M.H.; Elasaly, M.K.; Omar, S.A.; El-Nabarawi, M.A.; Shoueir, K.R. Wound healing activities of polyurethane modified chitosan nanofibers loaded with different concentrations of linezolid in an experimental model of diabetes. J. Drug Deliv. Sci. Technol. 2022, 67, 102982. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Ali, M.A.; Ahmed, O.S. To evaluate safety and efficacy of tedizolid phosphate in the management of several skin infections. Int. J. Res. Pharm. 2018, 1, 41–49. [Google Scholar] [CrossRef]
- Dou, J.L.; Jiang, Y.W.; Xie, J.Q.; Zhang, X.G. New is old, and old is new: Recent advances in antibiotic-based, antibiotic-free and ethnomedical treatments against methicillin-resistant Staphylococcus aureus wound infections. Int. J. Mol. Sci. 2016, 17, 617. [Google Scholar] [CrossRef] [Green Version]
- Fajardo, A.R.; Lopes, L.C.; Caleare, A.O.; Britta, E.A.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Silver sulfadiazine loaded chitosan/chondroitin sulfate films for a potential wound dressing application. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Hasselmann, J.; Kühme, T.; Acosta, S. Antibiotic prophylaxis with trimethoprim/sulfamethoxazole instead of cloxacillin fails to improve inguinal surgical site infection rate after vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 129–134. [Google Scholar] [CrossRef]
- Gjorevski, N.; Nikolaev, M.; Brown, T.E.; Mitrofanova, O.; Brandenberg, N.; DelRio, F.W.; Yavitt, F.M.; Liberali, P.; Anseth, K.S.; Lutolf, M.P. Tissue geometry drives deterministic organoid patterning. Science 2022, 375, eaaw9021. [Google Scholar] [CrossRef] [PubMed]
- ValadanTahbaz, S.; Azimi, L.; Asadian, M.; Lari, A.R. Evaluation of synergistic effect of tazobactam with meropenem and ciprofloxacin against multi-drug resistant Acinetobacter baumannii isolated from burn patients in Tehran. GMS Hyg. Infect. Control 2019, 14, Doc08. [Google Scholar] [CrossRef]
- Yang, M.; Hu, Z.; Hu, F. Nosocomial meningitis caused by Acinetobacter baumannii: Risk factors and their impact on patient outcomes and treatments. Future Microbiol. 2012, 7, 787–793. [Google Scholar] [CrossRef]
- NourianDehkordi, A.; MirahmadiBabaheydari, F.; Chehelgerdi, M.; RaeisiDehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef]
- Gonzales, K.A.U.; Fuchs, E. Skin and its regenerative powers: An alliance between stem cells and their niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [CrossRef]
- Azari, Z.; Nazarnezhad, S.; Webster, T.J.; Hoseini, S.J.; Brouki Milan, P.; Baino, F.; Kargozar, S. Stem cell-mediated angiogenesis in skin tissue engineering and wound healing. Wound Repair Regen. 2022, 30, 421–435. [Google Scholar] [CrossRef]
- Kamath, J.V.C.; Rana, A.C.; Chowdhury, A.R. Pro-healing effect of Cinnamomum zeylanicum bark. Phytother. Res. 2003, 17, 970–972. [Google Scholar] [CrossRef]
- Liang, J.; Cui, L.; Li, J.; Guan, S.; Zhang, K.; Li, J. Aloe vera: A medicinal plant used in skin wound healing. Tissue Eng. Part B Rev. 2021, 27, 455–474. [Google Scholar] [CrossRef]
- Teplicki, E.; Ma, Q.; Castillo, D.E.; Zarei, M.; Hustad, A.P.; Chen, J.; Li, J. The Effects of Aloe vera on Wound Healing in Cell Proliferation, Migration, and Viability. Wounds 2018, 30, 263–268. [Google Scholar] [PubMed]
- Hamman, J.H. Composition and applications of Aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef]
- RaesiVanani, A.; Mahdavinia, M.; Kalantari, H.; Khoshnood, S.; Shirani, M. Antifungal effect of the effect of Securigera securidaca L. vaginal gel on Candida species. Curr. Med. Mycol. 2019, 5, 31–35. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, S.R.; Chai, L.; Zhao, J.; Wang, Y.; Wang, Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. 1), S17–S29. [Google Scholar] [CrossRef]
- Nagulapalli Venkata, K.C.; Swaroop, A.; Bagchi, D.; Bishayee, A. A small plant with big benefits: Fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol. Nutr. Food Res. 2017, 61, 1600950. [Google Scholar] [CrossRef]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, S.M.; Leung, G.P.; Yu, P.H.; Chan, S.W. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2011, 19, 245–254. [Google Scholar] [CrossRef]
- Wei, W.L.; Zeng, R.; Gu, C.M.; Qu, Y.; Huang, L.F. Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J. Ethnopharmacol. 2016, 190, 116–141. [Google Scholar] [CrossRef]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Repair Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef]
- Li, Y.; Dong, M.; Wu, Z.; Huang, Y.; Qian, H.; Huang, C. Activity Screening of the Herb Caesalpinia sappan and an Analysis of Its Antitumor Effects. Evid. Based Complement. Altern. Med. 2021, 2021, 9939345. [Google Scholar] [CrossRef]
- Sugimoto, S.; Matsunami, K. Biological activity of Entada phaseoloides and Entada rheedei. J. Nat. Med. 2018, 72, 12–19. [Google Scholar] [CrossRef]
- Kiefer, D.; Pantuso, T. Panax ginseng. Am. Fam. Physician. 2003, 68, 1539–1542. [Google Scholar] [PubMed]
- Fang, Q.; Yao, Z.; Feng, L.; Liu, T.; Wei, S.; Xu, P.; Guo, R.; Cheng, B.; Wang, X. Antibiotic-loaded chitosan-gelatin scaffolds for infected seawater immersion wound healing. Int. J. Biol. Macromol. 2020, 159, 1140–1155. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lu, G.; Wu, Y.; Jirigala, E.; Xu, Y.; Ma, K.; Fu, X. Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J. Dermatol. Sci. 2012, 66, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, F. Core-shell chitosan microsphere with antimicrobial and vascularized functions for promoting skin wound healing. Mater. Des. 2021, 204, 109683. [Google Scholar] [CrossRef]
- Zhang, D.; Ouyang, Q.; Hu, Z.; Lu, S.; Quan, W.; Li, P.; Chen, Y.; Li, S. Catechol functionalized chitosan/active peptide microsphere hydrogel for skin wound healing. Int. J. Biol. Macromol. 2021, 173, 591–606. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed]
- MofazzalJahromi, M.; SahandiZangabad, P.; MoosaviBasri, S.M.; SahandiZangabad, K.; Ghamarypour, A.; Aref, A.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef] [PubMed]
- Likus, W.; Bajor, G.; Siemianowicz, K. Nanosilver—Does it have only one face? Acta Biochim. Pol. 2013, 60, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Yin, H.; Chen, X.; Chen, T.H.; Liu, H.M.; Rao, S.S.; Tan, Y.J.; Qian, Y.X.; Liu, Y.; Hu, X.K. Ångstrom-scale silver particle-embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation. Sci. Adv. 2020, 6, eaba0942. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, C.; Lan, M.; Guo, Q.; Du, X.; Zhou, S.; Cui, P.; Hong, T.; Jiang, P.; Wang, J.; et al. Antibacterial Photodynamic Gold Nanoparticles for Skin Infection. ACS Appl. Bio. Mater. 2021, 19, 3124–3132. [Google Scholar] [CrossRef]
- García, I.; Henriksen-Lacey, M.; Calvo, J.; de Aberasturi, D.J.; Paz, M.M.; Liz-Marzán, L.M. Size-Dependent Transport and Cytotoxicity of Mitomycin-Gold Nanoparticle Conjugates in 2D and 3D Mammalian Cell Models. Bioconjug. Chem. 2019, 30, 242–252. [Google Scholar] [CrossRef]
- Francesko, A.; Petkova, P.; Tzanov, T. Hydrogel Dressings for Advanced Wound Management. Curr. Med. Chem. 2018, 25, 5782–5797. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Dhaliwal, K.; Lopez, N. Hydrogel dressings and their application in burn wound care. Br. J. Community Nurs. 2018, 23 (Suppl. 9), S24–S27. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Zhang, Y.; Lin, W.; Ke, J.; Liu, J.; Zhang, L.; Liu, J. A balanced charged hydrogel with anti-biofouling and antioxidant properties for treatment of irradiation-induced skin injury. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112538. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Kharaziha, M.; Baidya, A.; Annabi, N. Rational Design of Immunomodulatory Hydrogels for Chronic Wound Healing. Adv. Mater. 2021, 33, e2100176. [Google Scholar] [CrossRef]
- Niladri, R.; Nabanita, S.; Petr, H.; Petr, S. Permeability and biocompatibility of novel medicated hydrogel wound dressings. Soft Mater. 2010, 8, 338–357. [Google Scholar] [CrossRef]
- Reimer, K.; Fleischer, W.; Brögmann, B.; Schreier, H.; Burkhard, P.; Lanzendörfer, A.; Gümbel, H.; Hoekstra, H.; Behrens-Baumann, W. Povidone-iodine liposomes—An overview. Dermatology 1997, 195 (Suppl. 2), 93–99. [Google Scholar] [CrossRef]
- Xu, H.L.; Chen, P.P.; ZhuGe, D.L.; Zhu, Q.Y.; Jin, B.H.; Shen, B.X.; Xiao, J.; Zhao, Y.Z. Liposomes with Silk Fibroin Hydrogel Core to Stabilize bFGF and Promote the Wound Healing of Mice with Deep Second-Degree Scald. Adv. Healthc. Mater. 2017, 6, 1700344. [Google Scholar] [CrossRef]
- Reimer, K.; Vogt, P.M.; Broegmann, B.; Hauser, J.; Rossbach, O.; Kramer, A.; Rudolph, P.; Bosse, B.; Schreier, H.; Fleischer, W. An innovative topical drug formulation for wound healing and infection treatment: In vitro and in vivo investigations of a povidone-iodine liposome hydrogel. Dermatology 2000, 201, 235–241. [Google Scholar] [CrossRef]
- Sağıroğlu, A.A.; Çelik, B.; Güler, E.M.; Koçyiğit, A.; Özer, Ö. Evaluation of wound healing potential of new composite liposomal films containing coenzyme Q10 and d-panthenyl triacetate as combinational treatment. Pharm. Dev. Technol. 2021, 26, 444–454. [Google Scholar] [CrossRef]
- Santos, A.C.; Rodrigues, D.; Sequeira, J.A.D.; Pereira, I.; Simões, A.; Costa, D.; Peixoto, D.; Costa, G.; Veiga, F. Nanotechnological breakthroughs in the development of topical phytocompounds-based formulations. Int. J. Pharm. 2019, 572, 118787. [Google Scholar] [CrossRef]
- Shakeel, F.; Alam, P.; Anwer, M.K.; Alanazi, S.A.; Alsarra, I.A.; Alqarni, M.H. Wound healing evaluation of self-nanoemulsifying drug delivery system containing Piper cubeba essential oil. 3 Biotech 2019, 9, 82. [Google Scholar] [CrossRef]
- Koshak, A.E.; Algandaby, M.M.; Mujallid, M.I.; Abdel-Naim, A.B.; Alhakamy, N.A.; Fahmy, U.A.; Alfarsi, A.; Badr-Eldin, S.M.; Neamatallah, T.; Nasrullah, M.Z.; et al. Wound Healing Activity of Opuntia ficus-indica Fixed Oil Formulated in a Self-Nanoemulsifying Formulation. Int. J. Nanomed. 2021, 16, 3889–3905. [Google Scholar] [CrossRef]
- Ponto, T.; Latter, G.; Luna, G.; Leite-Silva, V.R.; Wright, A.; Benson, H.A.E. Novel Self-Nano-Emulsifying Drug Delivery Systems Containing Astaxanthin for Topical Skin Delivery. Pharmaceutics 2021, 13, 649. [Google Scholar] [CrossRef]
- Khan, M.; Nadhman, A.; Sehgal, S.A.; Siraj, S.; Yasinzai, M.M. Formulation and characterization of a Self-Emulsifying Drug Delivery System (SEDDS) of curcumin for the topical application in cutaneous and mucocutaneous leishmaniasis. Curr. Top. Med. Chem. 2018, 18, 1603–1609. [Google Scholar] [CrossRef]
- Anand, S.; Pandey, P.; Begum, M.Y.; Chidambaram, K.; Arya, D.K.; Gupta, R.K.; Sankhwar, R.; Jaiswal, S.; Thakur, S.; Rajinikanth, P.S. Electrospun biomimetic multifunctional nanofibers loaded with ferulic acid for enhanced antimicrobial and wound-healing activities in STZ-Induced Diabetic Rats. Pharmaceuticals 2022, 15, 302. [Google Scholar] [CrossRef]
- Samadian, H.; Zamiri, S.; Ehterami, A. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in vivo studies. Sci. Rep. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Almasian, A.; Najafi, F.; Eftekhari, M.; Shams Ardekani, M.R.; Sharifzadeh, M.; Khanavi, M. Preparation of Polyurethane/Pluronic F127 Nanofibers Containing Peppermint Extract Loaded Gelatin Nanoparticles for Diabetic Wounds Healing: Characterization, in vitro, and in vivo Studies. Evid. Based Complement. Altern. Med. 2021, 2021, 6646702. [Google Scholar] [CrossRef]
- Grip, J.; Engstad, R.; Skjæveland, I.; Škalko-Basnet, N.; Isaksson, J.; Basnet, P.; Holsæter, A.M. Beta-glucan-loaded nanofiber dressing improves wound healing in diabetic mice. Eur. J. Pharm. Sci. 2018, 121, 269–280. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Qin, C.; Khan, A.U.R.; Zhang, W.; Mo, X. Silk fibroin/poly-(L-lactide-co-caprolactone) nanofiber scaffolds loaded with Huangbai Liniment to accelerate diabetic wound healing. Colloids Surf. B Biointerfaces 2021, 199, 111557. [Google Scholar] [CrossRef]
- Alzarea, A.I.; Alruwaili, N.K.; Ahmad, M.M.; Munir, M.U.; Butt, A.M.; Alrowaili, Z.A.; Shahari, M.S.B.; Almalki, Z.S.; Alqahtani, S.S.; Dolzhenko, A.V.; et al. Development and Characterization of Gentamicin-Loaded Arabinoxylan-Sodium Alginate Films as Antibacterial Wound Dressing. Int. J. Mol. Sci. 2022, 23, 2899. [Google Scholar] [CrossRef]
- Lv, F.; Wang, J.; Xu, P.; Han, Y.; Ma, H.; Xu, H.; Chen, S.; Chang, J.; Ke, Q.; Liu, M.; et al. A conducive bioceramic/polymer composite biomaterial for diabetic wound healing. Acta Biomater. 2017, 60, 128–143. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.Z. Sustained curcumin release from PLGA microspheres improves bone formation under diabetic conditions by inhibiting the reactive oxygen species production. Drug Des. Dev. Ther. 2018, 12, 1453–1466. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Eid, H.M.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Abdelgawad, M.A.; Rateb, M.E.; Ali, M.R.A.; Alsalahat, I.; Abou-Taleb, H.A. Bilosomes as a promising nanoplatform for oral delivery of an alkaloid nutraceutical: Improved pharmacokinetic profile and snowballed hypoglycemic effect in diabetic rats. Drug Deliv. 2022, 29, 2694–2704. [Google Scholar] [CrossRef]
- Ternullo, S.; Schulte Werning, L.V.; Holsæter, A.M.; Škalko-Basnet, N. Curcumin-in-Deformable Liposomes-in-Chitosan-Hydrogel as a Novel Wound Dressing. Pharmaceutics 2019, 12, 8. [Google Scholar] [CrossRef]
- Cui, M.D.; Pan, Z.H.; Pan, L.Q. DangguiBuxue Extract-Loaded Liposomes in Thermosensitive Gel Enhance In Vivo Dermal Wound Healing via Activation of the VEGF/PI3K/Akt and TGF-β/SmadsSignaling Pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 8407249. [Google Scholar] [CrossRef]
- Kalantari, K.; Mostafavi, E.; Afifi, A.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale. 2020, 12, 2268–2291. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Anwar, M.M.; Saeed, H. Nanomaterials for application in wound Healing: Current state-of-the-art and future perspectives. J. Polym. Res. 2022, 29, 91. [Google Scholar] [CrossRef]
- Souriyan-Reyhani pour, H.; Khajavi, R.; Yazdanshenas, M.E.; Zahedi, P.; Mirjalili, M. Cellulose acetate/poly(vinyl alcohol) hybrid fibrous mat containing tetracycline hydrochloride and phenytoin sodium: Morphology, drug release, antibacterial, and cell culture studies. J. Bioact. Compat. Polym. 2018, 33, 597–611. [Google Scholar] [CrossRef]
- Kong, Y.; Xu, R.; Darabi, M.A.; Zhong, W.; Luo, G.; Xing, M.M.Q.; Wu, J. Fast and safe fabrication of a free-standing chitosan/alginate nanomembrane to promote stem cell delivery and wound healing. Int. J. Nanomed. 2016, 11, 2543–2555. [Google Scholar] [CrossRef]
- Lohmann, N.; Schirmer, L.; Atallah, P.; Wandel, E.; Ferrer, R.A.; Werner, C.; Simon, J.C.; Franz, S.; Freudenberg, U. Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci. Transl. Med. 2017, 9, eaai9044. [Google Scholar] [CrossRef]
- Lu, K.J.; Wang, W.; Xu, X.L.; Jin, F.Y.; Qi, J.; Wang, X.J.; Kang, X.Q.; Zhu, M.L.; Huang, Q.L.; Yu, C.H.; et al. A dual deformable liposomal ointment functionalized with retinoic acid and epidermal growth factor for enhanced burn wound healing therapy. Biomater. Sci. 2019, 7, 2372–2382. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; Deng, Y.; Yuan, Z.; Shen, X.; Chen, M.; He, Y.; Peng, Z.; Hu, Y.; Cai, K. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J. Mater. Chem. B 2019, 7, 2534–2548. [Google Scholar] [CrossRef]
- Cao, L.; Shao, G.; Ren, F.; Yang, M.; Nie, Y.; Peng, Q.; Zhang, P. Cerium oxide nanoparticle-loaded polyvinyl alcohol nanogels delivery for wound healing care systems on surgery. Drug Deliv. 2021, 28, 390–399. [Google Scholar] [CrossRef]
- Ziv-Polat, O.; Topaz, M.; Brosh, T.; Margel, S. Enhancement of incisional wound healing by thrombin conjugated iron oxide nanoparticles. Biomaterials 2010, 31, 741–747. [Google Scholar] [CrossRef]
- Walker, R.M.; Gillespie, B.M.; Thalib, L.; Higgins, N.S.; Whitty, J.A. Foam dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2017, 10, CD011332. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, C.; Tan, R.; Zhang, J.; Fang, Q.; Jin, R.; Mi, X.; Sun, D.; Xue, Y.; Wang, Y.; et al. Artificial Intelligence-Assisted Bioinformatics, Microneedle, and Diabetic Wound Healing: A "New Deal" of an Old Drug. ACS Appl. Mater. Interfaces 2022, 14, 37396–37409. [Google Scholar] [CrossRef]
- Yao, S.; Wang, Y.; Chi, J.; Yu, Y.; Zhao, Y.; Luo, Y.; Wang, Y. Porous MOF Microneedle Array Patch with Photothermal Responsive Nitric Oxide Delivery for Wound Healing. Adv. Sci. 2022, 9, e2103449. [Google Scholar] [CrossRef]
- Shan, Y.; Tan, B.; Zhang, M.; Xie, X.; Liao, J. Restorative biodegradable two-layered hybrid microneedles for melanoma photothermal/chemo co-therapy and wound healing. J. Nanobiotechnol. 2022, 20, 238. [Google Scholar] [CrossRef]
- Sillmon, K.; Moran, C.; Shook, L.; Lawson, C.; Burfield, A.H. The Use of Prophylactic Foam Dressings for Prevention of Hospital-Acquired Pressure Injuries: A Systematic Review. J. Wound Ostomy Cont. Nurs. 2021, 48, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Sánchez, Á.; Kim, K.H.; Blasco-Morente, G.; Arias-Santiago, S. Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. NPJ Regen. Med. 2021, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Oh, Y.N.; Son, Y.R.; Kwon, B.; Park, J.H.; Gang, M.J.; Kim, B.W.; Kwon, H.J. Three-Dimensional Skin Tissue Printing with Human Skin Cell Lines and Mouse Skin-Derived Epidermal and Dermal Cells. J Microbiol. Biotechnol. 2022, 32, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Ngo, Z.H.; Sci, D.B.; Leavesley, D.; Liang, K. Recent Advances in the Design of Three-Dimensional and Bioprinted Scaffolds for Full-Thickness Wound Healing. Tissue Eng. Part B Rev. 2022, 28, 160–181. [Google Scholar] [CrossRef] [PubMed]
- Varkey, M.; Visscher, D.O.; van Zuijlen, P.P.M.; Atala, A.; Yoo, J.J. Skin bioprinting: The future of burn wound reconstruction? Burns. Trauma 2019, 7, 4. [Google Scholar] [CrossRef]
- Gupta, P.; Sheikh, A.; Abourehab, M.A.S.; Kesharwani, P. Amelioration of Full-Thickness Wound Using Hesperidin Loaded Dendrimer-Based Hydrogel Bandages. Biosensors 2022, 12, 462. [Google Scholar] [CrossRef]
- Vedhanayagam, M.; Unni Nair, B.; Sreeram, K.J. Collagen-ZnO Scaffolds for Wound Healing Applications: Role of Dendrimer Functionalization and Nanoparticle Morphology. ACS Appl. Bio. Mater. 2018, 1, 1942–1958. [Google Scholar] [CrossRef]
- Patrulea, V.; Borchard, G.; Jordan, O. An Update on Antimicrobial Peptides (AMPs) and Their Delivery Strategies for Wound Infections. Pharmaceutics 2020, 12, 840. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, W.; Xu, S.; Wei, J.; Lasaosa, F.L.; He, Y.; Mao, H.; BoleaBailo, R.M.; Kong, D.; Gu, Z. Bioinspired design of mannose-decorated globular lysine dendrimers promotes diabetic wound healing by orchestrating appropriate macrophage polarization. Biomaterials 2022, 280, 121323. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H. Polymeric Nanomaterials for Efficient Delivery of Antimicrobial Agents. Pharmaceutics 2021, 13, 2108. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, S.; Yu, T.; Wu, R.; Ren, Y.; van der Mei, H.C.; Liu, J.; Busscher, H.J. PAMAM dendrimers with dual-conjugated vancomycin and Ag-nanoparticles do not induce bacterial resistance and kill vancomycin-resistant Staphylococci. Acta Biomater. 2021, 123, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sayed, P.; Kaeppeli, A.; Siriwardena, T.; Darbre, T.; Perron, K.; Jafari, P.; Reymond, J.L.; Pioletti, D.P.; Applegate, L.A. Anti-Microbial Dendrimers against Multidrug-Resistant P. aeruginosa Enhance the Angiogenic Effect of Biological Burn-wound Bandages. Sci. Rep. 2016, 6, 22020. [Google Scholar] [CrossRef] [PubMed]
- Dongargaonkar, A.A.; Bowlin, G.L.; Yang, H. Electrospun blends of gelatin and gelatin-dendrimer conjugates as a wound-dressing and drug-delivery platform. Biomacromolecules 2013, 14, 4038–4045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, B.; Meng, X.; Sun, G.; Gao, C. Influences of acid-treated multiwalled carbon nanotubes on fibroblasts: Proliferation, adhesion, migration, and wound healing. Ann. Biomed. Eng. 2011, 39, 414–426. [Google Scholar] [CrossRef]
- Kittana, N.; Assali, M.; Abu-Rass, H.; Lutz, S.; Hindawi, R.; Ghannam, L.; Zakarneh, M.; Mousa, A. Enhancement of wound healing by single-wall/multi-wall carbon nanotubes complexed with chitosan. Int. J. Nanomed. 2018, 13, 7195–7206. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, G.; Chen, X.; Zhang, X.; Yu, J.; Liu, M.; Zhang, Y.; Wang, P. Degradation Behavior In Vitro of Carbon Nanotubes (CNTs)/Poly(lactic acid) (PLA) Composite Suture. Polymers 2019, 11, 1015. [Google Scholar] [CrossRef]
- Liu, J.; Ismail, N.A.; Yusoff, M.; Razali, M.H. Physicochemical Properties and Antibacterial Activity of Gellan Gum Incorporating Zinc Oxide/Carbon Nanotubes Bionanocomposite Film for Wound Healing. Bioinorg. Chem. Appl. 2022, 2022, 3158404. [Google Scholar] [CrossRef]
- Khalid, A.; Madni, A.; Raza, B.; Islam, M.U.; Hassan, A.; Ahmad, F.; Ali, H.; Khan, T.; Wahid, F. Multiwalled carbon nanotubes functionalized bacterial cellulose as an efficient healing material for diabetic wounds. Int. J. Biol. Macromol. 2022, 203, 256–267. [Google Scholar] [CrossRef]
- Chen, G.; Wu, Y.; Yu, D.; Li, R.; Luo, W.; Ma, G.; Zhang, C. Isoniazid-loaded chitosan/carbon nanotubes microspheres promote secondary wound healing of bone tuberculosis. J. Biomater. Appl. 2019, 33, 989–996. [Google Scholar] [CrossRef]
- Zhao, D.; Jing, H.; Li, X.; Zhao, W. Application of Nano-Composite Technology for Multi-Empty Carbon Nanotubes in Dressing Change Care. J. Nanosci. Nanotechnol. 2021, 21, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Wu, J.; Deng, M.; Wang, P.; Ji, G.; Wang, M.; Zhou, C.; Blum, N.T.; Zhang, W.; Shi, H.; et al. Multifunctional Magnesium Organic Framework-Based Microneedle Patch for Accelerating Diabetic Wound Healing. ACS Nano 2021, 15, 17842–17853. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H.; Guo, M.; Chu, J.; Gao, B.; He, B. Personalized and Programmable Microneedle Dressing for Promoting Wound Healing. Adv. Healthc. Mater. 2022, 11, e2101659. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Chi, J.; Sun, L.; Cai, L.; Fan, L.; Shao, C.; Shang, L.; Zhao, Y. Chinese herb microneedle patch for wound healing. Bioact. Mater. 2021, 6, 3507–3514. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.; He, B. Toward Efficient Wound Management: Bioinspired Microfluidic and Microneedle Patch. Small 2022, 19, e2206270. [Google Scholar] [CrossRef]
- Liu, A.; Wang, Q.; Zhao, Z.; Wu, R.; Wang, M.; Li, J.; Sun, K.; Sun, Z.; Lv, Z.; Xu, J.; et al. Nitric Oxide Nanomotor Driving Exosomes-Loaded Microneedles for Achilles Tendinopathy Healing. ACS Nano 2021, 15, 13339–13350. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, W.; Zhai, X.; Zhao, X.; Wang, J.; Weng, J.; Li, J.; Chen, X. An antibacterial and proangiogenic double-layer drug-loaded microneedle patch for accelerating diabetic wound healing. Biomater. Sci. 2023, 11, 533–541. [Google Scholar] [CrossRef]
- Xu, F.W.; Lv, Y.L.; Zhong, Y.F.; Xue, Y.N.; Wang, Y.; Zhang, L.Y.; Hu, X.; Tan, W.Q. Beneficial Effects of Green Tea EGCG on Skin Wound Healing: A Comprehensive Review. Molecules 2021, 26, 6123. [Google Scholar] [CrossRef]
- Barnum, L.; Samandari, M.; Schmidt, T.A.; Tamayol, A. Microneedle arrays for the treatment of chronic wounds. Expert Opin. Drug Deliv. 2020, 17, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Faraji Rad, Z.; Prewett, P.D.; Davies, G.J. An overview of microneedle applications, materials, and fabrication methods. Beilstein J. Nanotechnol. 2021, 12, 1034–1046. [Google Scholar] [CrossRef] [PubMed]



| Class | Name | Wound Dressing Material | Tested Strains | Administration | References |
|---|---|---|---|---|---|
| Macrolides | Clarithromycin Erythromycin | PVA hydrogels | Pseudomonas aeruginosa Staphylococcus aureus | Oral/Systemic/ Topical/ ophthalmic | [58] |
| Tetracycline | Tetracycline Chlortetracycline | Cotton fabric coated with chitosan-Poly (vinyl pyrrolidone)-PEG | E. coli S. aureus | Oral/Topical | [59] |
| Scaffolds with Collagen Microsphere with gelatin | E. coli S. aureus | Oral/Systemic/ Topical | [61] | ||
| Aminoglycosides | Streptomycin Neomycin | Wafers and film based on polymer Polyox/carrageenan | E. coli S. aureus P. aeruginosa | Oral/Topical/Systemic oral/topical | [66] [67] |
| poly(styrene sulfonic acid-co-maleic acid) (PSSA-MA)/polyvinyl alcohol (PVA) ion exchange nanofibres | E. coli S. aureus | ||||
| Fluoroquinolones | Norfloxacin Ciprofloxacin | Films and nanofibre mats of povidone Electrospun fibers based on thermoresponsive polymer poly(N-isopropylacrylamide), poly(L-lactic acid-co-ɛ-caprolactone) Hydrogels from 2-hydroxyethyl methacrylate/citraconic anhydride-modified collagen Films and nanofibre mats of povidine | E. coli Bacillus subtilis E. coli S. aureus S. aureus E.coli Bcillus subtilis | Topical Topical Topical | [69] [70] [71] |
| Formulation | Drug | Administration | Outcome | References |
|---|---|---|---|---|
| Nanofibre | Gentamicin sulphate (GS) | Topical application | It promotes cell adhesion and proliferation to scaffolds, and ultimately tissue regeneration and promotes healing process. | [129] |
| Nanofibre | Ferulic acid | Topically applied every day | Increased migration of cells to the wound site to fill the gap and increased proliferation causing rapid wound healing. | [130] |
| Nanofibre | Berberine | Topical treatment | Exhibited antibacterial activity against Gram-positive and Gram-negative bacterium. Animal studies on the STZ-induced diabetic rats demonstrated that the CA/Gel/Beri dressing enhanced the wound healing process. | [131] |
| Nanofibre | Peppermint | Topical dressing | Accelerated response and less inflammation and nanofibres showed potent wound healing activity for diabetic ulcers. | [132] |
| Nanofibre | Beta-glucan (βG) | Topically applies once a day | Enhanced maturation of granulation tissue and better healing process. | [133] |
| Nanofibre | Huangbai liniment (compound phellodendron liquid, CPL) | Topical treatment | It was also found that composite nanofibre membrane could reduce wound inflammation, down-regulate the expression of IL-1β and TNF-α inflammatory genes, and facilitate wound healing. | [134] |
| Nanofibre | Gentamicin salt (GEN) | Topical dressing | It has excellent antibacterial properties against Gram-negative E. coli, which is due to the unique properties of silver nanoparticles for antibacterial activity, and this composite has a good release profile for wound healing. | [135] |
| Nanofibre | Poly (caprolactone) (PCL) | Topical dressing once every day | It significantly promoted adhesion, proliferation and induced angiogenesis, collagen deposition, and re-epithelialisation in the wound sites of diabetic mice model, as well as inhibited inflammation reaction. | [136] |
| Hydrogel | Curcumin | Daily topical treatment | Shorten inflammatory process, prevents infection and re-epithelisation and promotes wound closer. | [137] |
| Liposome | Citicoline/chitosan | Topical treatment | Chitosan-coated liposomes containing citicoline have emerged as a potential approach for promoting the healing process in diabetic rats. However, the therapeutic effectiveness of the suggested approach in diabetic patients needs to be investigated. | [138] |
| Liposome | Curcumin | Topically applied once a day for 18 days | Curcumin-loaded liposomes in lysine–collagen hydrogel was found to be the most effective of the three formulations in promoting wound healing. Hence, this formulation can serve as a prototype for further development and has great potential as a smart wound dressing for the treatment of surgical wounds. | [139] |
| Liposome | DangguiBuxue | Topically applied | Remarkably accelerates wound closure, enhances hydroxyproline content in wound granulation tissue, promotes cutaneous wound healing by reducing the inflammatory response and improving fresh granulation tissue formation, and significantly increases the density of blood vessels and cell proliferation. | [140] |
| Nanoparticle | Silver | Topical treatment | Rapid healing and improved cosmetic appearance via reduction in wound inflammation and modulation of fibrogenic. | [141] |
| Nanoparticle | Zinc oxide (ZnO2) | Topical dressing | Had excellent anti-bacterial activity and rapid wound healing. | [142] |
| Nanomembrane | Triphala | Triphala PCL shows good broad spectrum of antimicrobial activity and biocompatibility and helps control wound infection and enhanced healing due to antioxidants of Triphala. | [143] | |
| Nanomembrane | Chitosan | Topical treatment | This nanomembrane serves as an excellent microenvironment for cell adhesion, migration, proliferation, and differentiation. An in vivo experiment with this nanomembrane was also conducted, showing that it has a great capability for stem cell delivery for skin tissue reconstruction. | [144] |
| Hydrogel | Glycosaminoglycan | Topical application | Promotion of tissue proliferation and regeneration of vascular vessels. | [138] |
| Deformable liposome | Curcumin | Daily topical treatment | CDLs in hydrogel preserved hydrogel’s bioadhesiveness to a higher degree than both NDLs and ADLs. In addition, CDLs-in-hydrogel enabled the most sustained skin penetration of curcumin and hence facilitates wound healing. | [145] |
| Liposomal ointment | Retinoic acid and growth factors | Topical application | Liposomal ointment on deep partial-thickness burn model stimulated wound closure (p < 0.001), promoted skin appendage formation and increased collagen production, thus improving healing quality. | [146] |
| Hydrogel nanoparticle | Copper (Cu) | Topical treatment | CuNP-comprised hydrogels exhibited a significant decrease in bacterial activity and promoted effective wound closure with negligible toxicity in our histological evolution. | [147] |
| Nanogel | Cerium oxide | Topical application | Showed significant antibacterial properties even at low absorptions and is effective at damage and scar production. | [148] |
| Nanoparticle | Thrombin | Topical treatment | The proportionate improvement in skin tensile strength after treatment with bound thrombin suggests that the novel thrombin conjugates may lessen surgical difficulties. | [149] |
| Microneedle | Trichostatin A, histone deacetylase 4 | Topical | The microneedle-mediated Trichostatin A patch has been shown to improve the healing of diabetic wounds by reducing inflammation, promoting tissue regeneration, and inhibiting histone deacetylase 4. | [150] |
| Metal–organic framework microneedle patch | Nitric oxide | Topical application | Delivering nitric oxide molecules more precisely and deeply into the wound site may be made possible by the integrated microneedle’s porous shape, increased specific surface area, and enough mechanical strength. | [151] |
| Microneedle | Curcumin nanodrugs/new Indocyanine Green/hyaluronic acid | Topical treatment | The two-layered microneedles platform has the potential to be used as a competitive technique for the treatment of melanoma since it can simultaneously remove the tumour and speed up wound healing. | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, R.; Pathak, K. Local Drug Delivery Strategies towards Wound Healing. Pharmaceutics 2023, 15, 634. https://doi.org/10.3390/pharmaceutics15020634
Tiwari R, Pathak K. Local Drug Delivery Strategies towards Wound Healing. Pharmaceutics. 2023; 15(2):634. https://doi.org/10.3390/pharmaceutics15020634
Chicago/Turabian StyleTiwari, Ruchi, and Kamla Pathak. 2023. "Local Drug Delivery Strategies towards Wound Healing" Pharmaceutics 15, no. 2: 634. https://doi.org/10.3390/pharmaceutics15020634
APA StyleTiwari, R., & Pathak, K. (2023). Local Drug Delivery Strategies towards Wound Healing. Pharmaceutics, 15(2), 634. https://doi.org/10.3390/pharmaceutics15020634