Exploring Synthetic Dihydrobenzofuran and Benzofuran Neolignans as Antiprotozoal Agents against Trypanosoma cruzi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Compounds 1–20
2.2. Animals
2.3. Parasites and Mammalian Cell Maintenance
2.4. Evaluation In Vitro of Antitrypanosomal Activity
2.4.1. Antitrypanosomal Activity against Drug-Sensitive T. cruzi Tulahuen Lac-Z Strain
2.4.2. Antitrypanosomal Activity against Moderately Drug-Resistant T. cruzi Y Strain
2.5. Determination of In Vitro Cytotoxicity and Hemolytic Activities
2.6. Computational Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Compounds 1–20
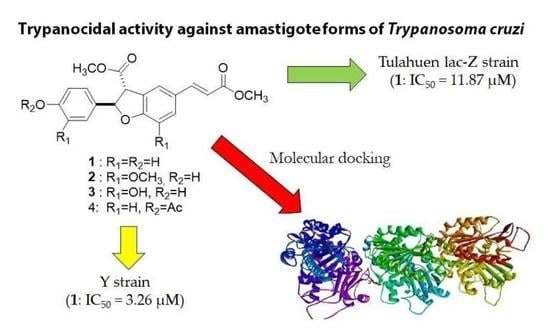
3.2. Antitrypanosomal Activity of Neolignans 1–20 against T. cruzi Amastigotes
3.3. Cytotoxic and Hemolytic Activities of Neolignans 1–4
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, P. Chagas disease: Quick facts. Nursing. 2020, 50, 13–15. [Google Scholar] [CrossRef] [PubMed]
- WHO. Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 3 January 2023).
- Pérez-Molina, J.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Kratz, J.M. Drug discovery for Chagas disease: A viewpoint. Acta Trop. 2019, 198, 105107. [Google Scholar] [CrossRef] [PubMed]
- Kratz, J.M.; Gonçalves, K.R.; Romera, L.M.; Moraes, C.B.; Cunha, P.B.; Schenkman, S.; Chatelain, E.; Sosa-Estani, S. The translational challenge in Chagas disease drug development. Mem. Inst. Oswaldo Cruz 2022, 117, e200501. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.S.N.; Perez-Molina, J.A.; Angheben, A.; Meymandi, S.K.; Sosa-Estani, S.; Molina, I. Critical analysis of Chagas disease treatment in different countries. Mem. Inst. Oswaldo Cruz 2022, 117, e210034. [Google Scholar] [CrossRef]
- Zingales, B.; Miles, M.A.; Campbell, D.A.; Tibayrenc, M.; Macedo, A.M.; Teixeira, M.M.; Schijman, A.G.; Llewellyn, M.S.; Lages-Silva, E.; Machado, C.R.; et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect. Genet. Evol. 2012, 12, 240–253. [Google Scholar] [CrossRef]
- Da Cunha, M.L.M.; Lima, L.H.S.; Ribeiro, L.O.; Pires, T.M.; Cembranelli, S.B.S.; Nunes, P.L.; Oliveira, S.M.A. Geographical distribution of DTUs from Trypanosoma cruzi isolated from human infections in Brazil: Systematic review. Braz. J. Develop. 2022, 8, 13334–13348. [Google Scholar] [CrossRef]
- Souza, T.K.M.; Westphalen, E.V.N.; Westphalen, S.D.R.; Taniguchi, H.H.; Elias, C.R.; Motoie, G.; Gava, R.; Pereira-Chioccola, V.L.; Novaes, C.T.G.; Carvalho, N.B.; et al. Genetic diversity of Trypanosoma cruzi strains isolated from chronic chagasic patients and non-human hosts in the state of São Paulo, Brazil. Mem. Inst. Oswaldo Cruz 2022, 117, e220125. [Google Scholar] [CrossRef]
- Velásquez-Ortiz, N.; Herrera, G.; Hernández, C.; Muñoz, M.; Ramírez, J.D. Discrete typing units of Trypanosoma cruzi: Geographical and biological distribution in the Americas. Sci. Data 2022, 9, 360. [Google Scholar] [CrossRef]
- Fonseca-Berzal, C.; Silva, P.B.; Silva, C.F.; Vasconcelos, M.; Batista, M.M.; Escario, J.A.; Aráni, V.J.; Gómez-Barrio, A.; Soeiro, M.N. Exploring the potential activity spectrum of two 5-nitroindazolinone prototypes on different Trypanosoma cruzi strains. Parasitol. Open 2015, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Souza, J.M.; Vieira, T.M.; Cândido, A.C.B.B.; Tezykja, D.Y.; Rao, S.; Albuquerque, S.; Crotti, A.E.M.; Siqueira-Neto, J.L.; Magalhães, L.G. In vitro anti-Trypanosoma cruzi activity enhacement of curcumin by its monoketone tetramethoxy analog diveratralacetone. Curr. Res. Parasitol. Vector Borne Dis. 2021, 1, e10003. [Google Scholar] [CrossRef]
- Amaral, M.; de Souza, F.S.; Silva, T.A.C.; Júnior, A.J.G.; Taniwaki, N.N.; Johns, D.M.; Lago, J.H.G.; Anderson, E.A.; Tempone, A.G. A semi-synthetic neolignan derivative from dihydrodieugenol B selectively affects the bioenergetic system of Leishmania infantum and inhibits cell division. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miert, S.V.; Dyck, S.V.; Schmidt, T.J.; Brun, R.; Vlietinck, A.; Lemie, G.; Pieters, L. Antileishmanial activity, cytotoxicity and QSAR analysis of synthetic dihydrobenzofuran lignans and related benzofurans. Bioorg. Med. Chem. 2005, 13, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Pellizzaro-Rocha, K.J.; Veiga-Santos, P.; Lazarin-Bidóia, D.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Ximenes, V.F.; Silva, S.O.; Nakamura, C.V. Trypanocidal action of eupomatenoid-5 is related to mitochondrion dysfunction and oxidative damage in Trypanosoma cruzi. Microbes Infect. 2011, 13, 1018–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.C.; Magalhães, L.G.; Gonçalves, U.O.; Luz, P.P.; Moraes, A.C.G.; Rodrigues, V.; Matta-Guedes, P.M.; Silva-Filho, A.A.; Cunha, W.R.; Bastos, J.K.; et al. Schistosomicidal and trypanocidal structure-activity relationships for (±)-licarin A and its (-)- and (+)-enantiomers. Phytochemistry 2011, 72, 1424–1430. [Google Scholar] [CrossRef]
- Oliveira, L.G.C.; Brito, L.M.; Alves, M.M.M.; Amorim, L.V.; Sobrinho-Júnior, E.P.C.; Carvalho, C.E.S.; Rodrigues, K.A.F.; Arcanjo, D.D.R.; Citó, A.M.G.L.; Carvalho, F.A.A. In vitro effects of the neolignan 2,3-dihydrobenzofuran against Leishmania amazonensis. Basic. Clin. Pharmacol. Toxicol. 2017, 120, 52–58. [Google Scholar] [CrossRef]
- Dias, H.J.; Patrocínio, A.B.; Pagotti, M.C.; Fukui, M.J.; Rodrigues, V.; Magalhães, L.G.; Crotti, A.E.M. Schistosomicidal activity of dihydrobenzofuran neolignans. Chem. Biodiv. 2018, 15, e1800134. [Google Scholar] [CrossRef]
- Medeiros, T.C.T.; Dias, H.J.; Silva, E.O.; Fukui, M.J.; Soares, A.C.F.; Kar, T.; Heleno, V.C.G.; Donate, P.M.; Parreira, R.L.T.; Crotti, A.E.M. Detailed 1H and 13C NMR spectral data assignment for two dihydrobenzofuran neolignans. J. Braz. Chem. Soc. 2016, 27, 136–143. [Google Scholar] [CrossRef]
- Dias, H.J.; Rodrigues, M.L.; Crotti, A.E.M. Optimization of the reaction conditions for the synthesis of dihydrobenzofuran neolignans. J. Braz. Chem. Soc. 2021, 32, 20–28. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Wu, C.H. Synthesis of 5-(3-hydroxypropyl)-7-methoxy-2-(3′-methoxy-4′-hydroxyphenyl)-3-benzo[b]furancarbaldehyde, a novel adenosine A1 receptor ligand from the root of Salvia miltiorrhiza. J. Nat. Prod. 1996, 59, 625–628. [Google Scholar] [CrossRef]
- Maeda, S.; Masuda, H.; Tokoroyama, T. Studies on the preparation of bioactive lignans by oxidative coupling reaction. V. Oxidative coupling reaction of methyl (E)-3-(2-hydroxyphenyl)propenoate derivatives and lipid peroxidation inhibitory effects of the produced lignans. Chem. Pharm. Bull. 1994, 42, 2536–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieters, L.; Dyck, S.V.; Gao, M.; Bai, R.; Hamel, E.; Vietinck, A.; Lemière, G. Synthesis and biological evaluation of dihydrobenzofuran lignans and related compounds as potential antitumor agents that inhibit tubulin polymerization. J. Med. Chem. 1999, 42, 5475–5481. [Google Scholar] [CrossRef] [PubMed]
- Felix, A.M. Cleavage of protecting groups with boron tribromide. J. Org. Chem. 1974, 39, 1427–1429. [Google Scholar] [CrossRef]
- Maia, P.L.S.; Carneiro, Z.A.; Lopes, C.D.; Oliveira, C.G.; Silva, J.S.; de Albuquerque, S.; Hagenbach, A.; Gust, R.; Delon, V.M.; Abram, U. Organometallic gold(iii) complexes with hybrid SNS-donating thiosemicarbazone ligands: Cytotoxicity and anti-Trypanosoma cruzi activity. Dalton Trans. 2017, 46, 2559–2571. [Google Scholar] [CrossRef]
- Dias, L.C.; Dessoy, M.A.; Silva, J.J.N.; Thiemann, O.H.; Oliva, G.; Andricopulo, A.D. Chemotherapy of chagas’ disease: State of the art and perspectives for the development of new drugs. Quim. Nova 2009, 32, 2444–2447. [Google Scholar] [CrossRef]
- Soeiro, M.N.; de Souza, E.M.; da Silva, C.F.; Batista, D.G.; Batista, M.M.; Pavão, B.P.; Araújo, J.S.; Aiub, C.A.F.; da Silva, P.B.; Lionel, J.; et al. In vitro and in vivo studies of the antiparasitic activity of sterol 14α-demethylase (CYP51) inhibitor VNI against drugresistant strains of Trypanosoma cruzi. Antimicrob. Agents Chemother. 2013, 57, 4151–4163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, L.H.; Nussenzweig, V. Sobre uma cepa de Trypanosoma cruzi altamente virulenta para o camundongo branco. Fol. Clin. Biol. 1953, 20, 191–207. [Google Scholar]
- Bettiol, E.; Samanovic, M.; Murkin, A.S.; Raper, J.; Buckner, F.; Rodriguez, A. Identification of three classes of heteroaromatic compounds with activity against intracellular Trypanosoma cruzi by chemical library screening. PLoS Negl. Trop. Dis. 2009, 3, e384. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.R. Standartization of a Colorimetric Method for Evaluation of Substances Biological Activity on Tachyzoite Forms of Toxoplasma gondii, With Evaluation of Acid Triterpenes on Parasite. Master’s Thesis, Faculty of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, Ribeirão Preto, SP, Brazil, 2009. [Google Scholar]
- Ag, L.C. Technical reference guide protocol for performing a trypan blue viability test. BioResearch 2012, 21, 2–3. [Google Scholar]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2015, 111. [Google Scholar] [CrossRef]
- Kaplum, V.; Cogo, J.; Sangi, D.P.; Ueda-Nakamura, T.; Corrêa, A.G.; Nakamura, C.V. In vitro and in vivo activities of 2,3-diarylsubstituted quinoxaline derivatives against Leishmania amazonensis. Antimicrob. Agents Chemother. 2016, 60, 3433–3444. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Schwede, T. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [Green Version]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2011, 27, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Kleywegt, G.J.; Jones, T.A. Phi/Psi-chology: Ramachandran revisited. Structure 1996, 4, 1395–1400. [Google Scholar] [CrossRef] [Green Version]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein-ligand docking using gold. Proteins 2003, 52, 609–623. [Google Scholar] [CrossRef]
- Dias, H.J.; Vieira, T.M.; Silva, E.O.; Crotti, A.E.M. Dihydrobenzofuran neolignans: An overview of their chemistry and biological activities. In Benzofuran: Production and Applications; Barros, M.S., Ed.; Nova Publisher: Hauppauge, NY, USA, 2020; pp. 31–116. [Google Scholar]
- Abe, F.; Nagafuji, S.; Yamauchi, T.; Okabe, H.; Maki, J.; Higo, H.; Akahane, H.; Aguilar, A.; Jiménez-Estrada, M.; Reyes-Chilpa, R. Trypanocidal constituents in plants 1. Evaluation of some Mexican plants for their trypanocidal activity and active constituents in Guaco, roots of Aristolochia taliscana. Biol. Pharm. Bull. 2002, 25, 1188–1191. [Google Scholar] [CrossRef] [Green Version]
- Luize, P.S.; Ueda-Nakamura, T.; Filho, B.P.D.; Cortez, D.A.G.; Nakamura, C.V. Activity of neolignans isolated from Piper regnellii (MIQ.) C. DC. var. pallescens (C. DC.) YUNCK against Trypanosoma cruzi. Biol. Pharm. Bull. 2006, 29, 2126–2130. [Google Scholar] [CrossRef] [Green Version]
- Martins, A.V.; Gomes, A.P.; Mendonça, E.G.; Fietto, J.L.R.; Santana, L.A.; Oliveira, M.G.A.; Geller, M.; Santos, R.F.; Vitorino, R.R.; Siqueira-Batista, R. Biology of Trypanosoma cruzi: An update. Infectio 2012, 16, 45–58. [Google Scholar] [CrossRef] [Green Version]
- Luize, P.S.; Ueda-Nakamura, T.; Dias Filho, B.P.; Cortez, D.A.G.; Morgado-Díaz, J.A.; Souza, W.; Nakamura, C.V. Ultrastructural alterations induced by the neolignan dihydrobenzofuranic eupomatenoid-5 on epimastigote and amastigote forms of Trypanosoma cruzi. Parasitol. Res. 2006, 100, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, K.; Burrows, J.N.; Duncan, K.; van Huijsduijnen, R.H.; Kaneko, T.; Kita, K.; Mombray, C.E.; Schmatz, D.; Warner, P.; Sligsby, B.T. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, E. Chagas disease drug discovery: Toward a new era. J. Biomol. Screen. 2015, 201, 22–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.A.; Passarini, G.M.; Martinez, L.N.; Fialho, S.N.; Sales-Júnior, P.A.; Fokoue, H.H.; Kato, M.J.; Teles, C.B.G.; Kuehn, C.C. In vitro of tripanocid activity of naturals amides and their respective synthetic analogs. S. Am. J. Basic Ed. Techn. Technol. 2020, 7, 186–197. [Google Scholar]
- Maia, M.S.; Andrade, R.S.; Sousa, J.M.S.; Sousa, N.F.; Rodrigues, G.C.S.; Menezes, R.P.B.; Silva, M.S.; Tavares, J.F.; Rodrigues, K.A.F.; Scotti, L.; et al. Virtual screening based on ligand and structure with in vitro assessment of neolignans against Trypanosoma cruzi. J. Braz. Chem. Soc. 2022, 15, 17. [Google Scholar] [CrossRef]
- Ogindo, C.O.; Khraiwesh, M.H.; George, M.; Brandy, Y.; Brandy, N.; Gugssa, A.; Ashraf, M.; Abbas, M.; Southerland, W.M.; Lee, C.M.; et al. Novel drug design for Chagas disease via targeting Trypanosoma cruzi tubulin: Homology modeling and binding pocket prediction on Trypanosoma cruzi tubulin polymerization inhibition by naphthoquinone derivatives. Bioorg. Med. Chem. 2016, 24, 3849–3855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborti, S.; Das, L.; Kapoor, N.; Das, A.; Dwivedi, V.; Poddar, A.; Chakraborti, G.; Janik, M.; Basu, G.; Panda, D.; et al. Curcumin recognizes a unique binding site of tubulin. J. Med. Chem. 2011, 54, 6183–6196. [Google Scholar] [CrossRef] [PubMed]
- Sueth-Santiago, V.; Moraes, J.B.B.; Alves, E.S.S.; Vannier-Santos, M.A.; Freire-de-Lima, C.G.; Castro, R.N.; Mendes-Silva, G.P.; Del Cistia, C.N.; Magalhães, L.G.; Andricopulo, A.D.; et al. The effectiveness of natural diarylheptanoids against Trypanosoma cruzi: Cytotoxicity, ultrastructural alterations and molecular modeling studies. PLoS ONE 2016, 11, e0162926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prota, A.E.; Danel, F.; Bachmann, F.; Bargsten, K.; Buey, R.M.; Pohlmann, J.; Reinelt, S.; Lane, H.; Steinmetz, M.O. The novel microtubule-destabilizing drug BAL27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization. J. Mol. Biol. 2014, 426, 1848–1860. [Google Scholar] [CrossRef]
- Ravelli, R.B.G.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, M.; Rotgans, B.A.; Strong, A.; Liang, D.; Ni, G.; Limpanont, Y.; Ramasoota, P.; McManus, D.P.; Cummins, S.F. Proteomic analysis of the Schistosoma mansoni miracidium. PLoS ONE 2016, 11, e0147247. [Google Scholar] [CrossRef] [Green Version]


| Compound | Tulahuen Lac-Z Strain | Y Strain |
|---|---|---|
| 1 | 11.87 (9.50–14.83) | 3.26 (1.56–6.80) |
| 2 | 7.96 (6.36–9.97) | >25 |
| 3 | 16.16 (11.55–22.63) | >25 |
| 4 | 21.42 (17.55–26.14) | >25 |
| 5 | 31.58 (25.5–39.12) | ND |
| 6 | 229 (219.7–238.8) | ND |
| 7 | 75.0 (58.51–95.15) | ND |
| 8 | 119.0 (112.2–126.2) | ND |
| 9 | 246.6 (196.9–308.7) | ND |
| 10 | 176.9 (117.8–265.8) | ND |
| 11 | 70.05 (65.74–74.64) | ND |
| 12 | 288.7 (282.8–292.7) | ND |
| 13 | 79.26 (73.74–85.2) | ND |
| 14 | 134.1 (92.65–194.2) | ND |
| 15 | 148.8 (100.8–219.6) | ND |
| 16 | 71.46 (67.58–75.57) | ND |
| 17 | 72.27 (66.14–78.97) | ND |
| 18 | 288.8 (279.3–298.6) | ND |
| 19 | 300.7 (296.5–304.9) | ND |
| 20 | 291.6 (286.4–297.0) | ND |
| BZN | 2.15 (0.04–9.082) | 3.56 (1.20–5.48) |
| DBN | CC50 | SI | CH50 | ||
|---|---|---|---|---|---|
| Murine Spleen (24 h) | LLC-MK2 (72 h) | Tulahuen Lac-Z Strain | Y Strain | Erythrocytes | |
| 1 | 49.87 (37.21–66.84) | 70.86 (43.03–116.7) | 5.96 | 21.73 | >100 |
| 2 | 34.20 (21.74–53.79) | 67.59 (46.11–99.09) | 8.49 | ND | >100 |
| 3 | <1.95 | 30.76 (22.0–43.01) | 1.90 | ND | >100 |
| 4 | 51.09 (40.22–64.91) | 67.50 (50.34–90.51) | 3.15 | ND | >100 |
| BZN | 1.81 (0.82–4.01) | 43.21 (41.87–44.55) | 20.05 | 12.13 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagotti, M.C.; Dias, H.J.; Candido, A.C.B.B.; Oliveira, T.A.S.; Borges, A.; Oliveira, N.D.; Lopes, C.D.; Orenha, R.P.; Parreira, R.L.T.; Crotti, A.E.M.; et al. Exploring Synthetic Dihydrobenzofuran and Benzofuran Neolignans as Antiprotozoal Agents against Trypanosoma cruzi. Pharmaceutics 2023, 15, 754. https://doi.org/10.3390/pharmaceutics15030754
Pagotti MC, Dias HJ, Candido ACBB, Oliveira TAS, Borges A, Oliveira ND, Lopes CD, Orenha RP, Parreira RLT, Crotti AEM, et al. Exploring Synthetic Dihydrobenzofuran and Benzofuran Neolignans as Antiprotozoal Agents against Trypanosoma cruzi. Pharmaceutics. 2023; 15(3):754. https://doi.org/10.3390/pharmaceutics15030754
Chicago/Turabian StylePagotti, Mariana C., Herbert J. Dias, Ana Carolina B. B. Candido, Thaís A. S. Oliveira, Alexandre Borges, Nicoli D. Oliveira, Carla D. Lopes, Renato P. Orenha, Renato L. T. Parreira, Antônio E. M. Crotti, and et al. 2023. "Exploring Synthetic Dihydrobenzofuran and Benzofuran Neolignans as Antiprotozoal Agents against Trypanosoma cruzi" Pharmaceutics 15, no. 3: 754. https://doi.org/10.3390/pharmaceutics15030754
APA StylePagotti, M. C., Dias, H. J., Candido, A. C. B. B., Oliveira, T. A. S., Borges, A., Oliveira, N. D., Lopes, C. D., Orenha, R. P., Parreira, R. L. T., Crotti, A. E. M., & Magalhães, L. G. (2023). Exploring Synthetic Dihydrobenzofuran and Benzofuran Neolignans as Antiprotozoal Agents against Trypanosoma cruzi. Pharmaceutics, 15(3), 754. https://doi.org/10.3390/pharmaceutics15030754