Modulation of Macrophage Function by Bioactive Wound Dressings with an Emphasis on Extracellular Matrix-Based Scaffolds and Nanofibrous Composites
Abstract
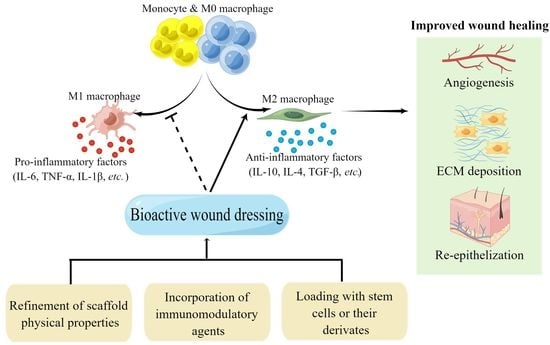
:1. Introduction
2. Roles of Macrophage in Normal Skin Wound Healing
3. Macrophage Dysfunction in Impaired or Chronic Wounds
4. Strategies to Regulate Macrophage Function by Bioactive Scaffolds
4.1. Refinement of Scaffold Physical Properties
4.1.1. Pore Size
4.1.2. Fiber Diameter
4.1.3. Stiffness
4.1.4. Topography
4.2. Incorporation of Immunomodulatory Agents
4.2.1. Cytokines
4.2.2. Chemical Compounds
4.3. Loading with Stem Cells or Their Derivates
4.3.1. Stem Cells
4.3.2. Stem Cells Derivates
5. Different Types of Bioactive Wound Dressings to Modulate Macrophage Function
5.1. ECM-Based Scaffolds
5.2. Nanofibrous Composites
5.3. Other Bioactive Wound Dressings
5.3.1. Nanoparticle Loaded Scaffolds
5.3.2. Hydrogel Wound Dressings
6. Future Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
References
- Huang, Y.-Z.; Gou, M.; Da, L.-C.; Zhang, M.W.-Q.; Xie, H.-Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Bai, X.; Dai, X.; Li, Y. The biological processes during wound healing. Regen. Med. 2021, 16, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, J.; Chen, H.; Shi, X.; Wang, X.; Zhu, Y.; Tan, Z. The topography of fibrous scaffolds modulates the paracrine function of Ad-MSCs in the regeneration of skin tissues. Biomater. Sci. 2019, 7, 4248–4259. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Pathalamuthu, P.; Siddharthan, A.; Giridev, V.R.; Victoria, V.; Thangam, R.; Sivasubramanian, S.; Savariar, V.; Hemamalini, T. Enhanced performance of Aloe vera incorporated chitosan-polyethylene oxide electrospun wound scaffold produced using novel Spirograph based collector assembly. Int. J. Biol. Macromol. 2019, 140, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-F.; Chen, C.-H.; Kao, H.-H.; Govindaraju, D.T.; Dash, B.S.; Chen, J.-P. PLGA/Gelatin/Hyaluronic Acid Fibrous Membrane Scaffold for Therapeutic Delivery of Adipose-Derived Stem Cells to Promote Wound Healing. Biomedicines 2022, 10, 2902. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, H.; Pan, X.; Zhang, C.; Zhang, K.; Chen, Z.; Dong, W.; Xie, A.; Qi, X. Dendritic Hydrogels with Robust Inherent Antibacterial Properties for Promoting Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 11144–11155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xia, S.; Weng, T.; Yang, M.; Shao, J.; Zhang, M.; Wang, J.; Xu, P.; Wei, J.; Jin, R.; et al. Antibacterial coaxial hydro-membranes accelerate diabetic wound healing by tuning surface immunomodulatory functions. Mater. Today Bio 2022, 16, 100395. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.-J.; Lv, Z.-Y.; Liu, K.; Meng, C.-X.; Zou, B.; Li, K.-Y.; Liu, F.-Z.; Zhang, B. The observed difference of macrophage phenotype on different surface roughness of mineralized collagen. Regen. Biomater. 2020, 7, 203–211. [Google Scholar] [CrossRef]
- Kang, H.; Jung, H.J.; Kim, S.K.; Wong, D.S.H.; Lin, S.; Li, G.; Dravid, V.P.; Bian, L. Magnetic Manipulation of Reversible Nanocaging Controls In Vivo Adhesion and Polarization of Macrophages. ACS Nano 2018, 12, 5978–5994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Zhang, X.; Zhu, D.; Qi, X.; Cao, X.; Fang, Y.; Che, Y.; Han, Z.-C.; He, Z.-X.; et al. Prostaglandin E2 hydrogel improves cutaneous wound healing via M2 macrophages polarization. Theranostics 2018, 8, 5348–5361. [Google Scholar] [CrossRef] [PubMed]
- Whelan, D.S.; Caplice, N.M.; Clover, A.J.P. Mesenchymal stromal cell derived CCL2 is required for accelerated wound healing. Sci. Rep. 2020, 10, 2642. [Google Scholar] [CrossRef]
- Gonzalez, A.C.D.O.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Kim, S.Y.; Nair, M.G. Macrophages in wound healing: Activation and plasticity. Immunol. Cell Biol. 2019, 97, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Su, Y.; Richmond, A. Chemokine Regulation of Neutrophil Infiltration of Skin Wounds. Adv. Wound Care 2015, 4, 631–640. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. 2021, 236, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Etich, J.; Koch, M.; Wagener, R.; Zaucke, F.; Fabri, M.; Brachvogel, B. Gene Expression Profiling of the Extracellular Matrix Signature in Macrophages of Different Activation Status: Relevance for Skin Wound Healing. Int. J. Mol. Sci. 2019, 20, 5086. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef]
- Sorokin, L.M. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010, 10, 712–723. [Google Scholar] [CrossRef]
- Mokarram, N.; Merchant, A.; Mukhatyar, V.; Patel, G.; Bellamkonda, R.V. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials 2012, 33, 8793–8801. [Google Scholar] [CrossRef]
- Madden, L.R.; Mortisen, D.J.; Sussman, E.M.; Dupras, S.K.; Fugate, J.A.; Cuy, J.L.; Hauch, K.D.; Laflamme, M.A.; Murry, C.E.; Ratner, B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 15211–15216. [Google Scholar] [CrossRef]
- Boddupalli, A.; Zhu, L.; Bratlie, K.M. Methods for Implant Acceptance and Wound Healing: Material Selection and Implant Location Modulate Macrophage and Fibroblast Phenotypes. Adv. Health Mater. 2016, 5, 2575–2594. [Google Scholar] [CrossRef]
- Boersema, G.S.; Grotenhuis, N.; Bayon, Y.; Lange, J.F.; Bastiaansen-Jenniskens, Y.M. The Effect of Biomaterials Used for Tissue Regeneration Purposes on Polarization of Macrophages. BioResearch Open Access 2016, 5, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef] [PubMed]
- Bartneck, M.; Schulte, V.A.; Paul, N.E.; Diez, M.; Lensen, M.C.; Zwadlo-Klarwasser, G. Induction of specific macrophage subtypes by defined micro-patterned structures. Acta Biomater. 2010, 6, 3864–3872. [Google Scholar] [CrossRef] [PubMed]
- Junge, K.; Binnebösel, M.; Von Trotha, K.T.; Rosch, R.; Klinge, U.; Neumann, U.P.; Jansen, P.L. Mesh biocompatibility: Effects of cellular inflammation and tissue remodelling. Langenbeck’s Arch. Surg. 2011, 397, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; He, X.-T.; Wang, J.; Wu, R.-X.; Xu, X.-Y.; Hong, Y.-L.; Tian, B.-M.; Chen, F.-M. Pore size-mediated macrophage M1-to-M2 transition influences new vessel formation within the compartment of a scaffold. Appl. Mater. Today 2020, 18, 100466. [Google Scholar] [CrossRef]
- Sussman, E.M.; Halpin, M.C.; Muster, J.; Moon, R.T.; Ratner, B.D. Porous Implants Modulate Healing and Induce Shifts in Local Macrophage Polarization in the Foreign Body Reaction. Ann. Biomed. Eng. 2014, 42, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Farrell, B.J.; Prilutsky, B.I.; Ritter, J.M.; Kelley, S.; Popat, K.C.; Pitkin, M. Effects of pore size, implantation time, and nano-surface properties on rat skin ingrowth into percutaneous porous titanium implants. J. Biomed. Mater. Res. Part A 2013, 102, 1305–1315. [Google Scholar] [CrossRef]
- Li, Z.; Bratlie, K.M. The Influence of Polysaccharides-Based Material on Macrophage Phenotypes. Macromol. Biosci. 2021, 21, e2100031. [Google Scholar] [CrossRef]
- Abebayehu, D.; Spence, A.J.; McClure, M.J.; Haque, T.T.; Rivera, K.O.; Ryan, J.J. Polymer scaffold architecture is a key determinant in mast cell inflammatory and angiogenic responses. J. Biomed. Mater. Res. Part A 2019, 107, 884–892. [Google Scholar] [CrossRef]
- Saino, E.; Focarete, M.L.; Gualandi, C.; Emanuele, E.; Cornaglia, A.I.; Imbriani, M.; Visai, L. Effect of Electrospun Fiber Diameter and Alignment on Macrophage Activation and Secretion of Proinflammatory Cytokines and Chemokines. Biomacromolecules 2011, 12, 1900–1911. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, Y.; Wang, J.; Yang, X.; Wu, Y.; Wang, K.; Gao, X.; Li, D.; Li, Y.; Zheng, X.-L.; et al. The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 2014, 35, 5700–5710. [Google Scholar] [CrossRef] [PubMed]
- Horii, T.; Tsujimoto, H.; Hagiwara, A.; Isogai, N.; Sueyoshi, Y.; Oe, Y.; Kageyama, S.; Yoshida, T.; Kobayashi, K.; Minato, H.; et al. Effects of Fiber Diameter and Spacing Size of an Artificial Scaffold on the In Vivo Cellular Response and Tissue Remodeling. ACS Appl. Bio Mater. 2021, 4, 6924–6936. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhang, Y.; Lim, C.T. Fabrication of Large Pores in Electrospun Nanofibrous Scaffolds for Cellular Infiltration: A Review. Tissue Eng. Part B Rev. 2012, 18, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Duann, P.; Lin, P.-H. Wound Matrix Stiffness Imposes on Macrophage Activation. Methods Mol. Biol. 2020, 2193, 111–120. [Google Scholar] [CrossRef]
- Vasse, G.F.; Nizamoglu, M.; Heijink, I.H.; Schlepütz, M.; Rijn, P.; Thomas, M.J.; Burgess, J.K.; Melgert, B.N. Macrophage–stroma interactions in fibrosis: Biochemical, biophysical, and cellular perspectives. J. Pathol. 2021, 254, 344–357. [Google Scholar] [CrossRef]
- Sridharan, R.; Cavanagh, B.; Cameron, A.R.; Kelly, D.J.; O’Brien, F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019, 89, 47–59. [Google Scholar] [CrossRef]
- Zhuang, Z.; Zhang, Y.; Sun, S.; Li, Q.; Chen, K.; An, C.; Wang, L.; Beucken, J.J.J.P.V.D.; Wang, H. Control of Matrix Stiffness Using Methacrylate–Gelatin Hydrogels for a Macrophage-Mediated Inflammatory Response. ACS Biomater. Sci. Eng. 2020, 6, 3091–3102. [Google Scholar] [CrossRef]
- Meli, V.S.; Atcha, H.; Veerasubramanian, P.K.; Nagalla, R.R.; Luu, T.U.; Chen, E.Y.; Guerrero-Juarez, C.F.; Yamaga, K.; Pandori, W.; Hsieh, J.Y.; et al. YAP-mediated mechanotransduction tunes the macrophage inflammatory response. Sci. Adv. 2020, 6, eabb8471. [Google Scholar] [CrossRef]
- Hsieh, J.Y.; Keating, M.T.; Smith, T.D.; Meli, V.S.; Botvinick, E.L.; Liu, W.F. Matrix crosslinking enhances macrophage adhesion, migration, and inflammatory activation. APL Bioeng. 2019, 3, 016103. [Google Scholar] [CrossRef]
- He, H.; Xiao, Z.; Zhou, Y.; Chen, A.; Xuan, X.; Li, Y.; Guo, X.; Zheng, J.; Xiao, J.; Wu, J. Zwitterionic poly(sulfobetaine methacrylate) hydrogels with optimal mechanical properties for improving wound healing in vivo. J. Mater. Chem. B 2019, 7, 1697–1707. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Li, Y.; Zhang, N.; Gribskov, M.; Zhang, X.; Lin, M.; Shao, D.; Zhang, C.; Dai, L.; et al. miRNA-mediated macrophage behaviors responding to matrix stiffness and ox-LDL. J. Cell. Physiol. 2020, 235, 6139–6153. [Google Scholar] [CrossRef]
- Gruber, E.; Heyward, C.; Cameron, J.; Leifer, C. Toll-like receptor signaling in macrophages is regulated by extracellular substrate stiffness and Rho-associated coiled-coil kinase (ROCK1/2). Int. Immunol. 2018, 30, 267–278. [Google Scholar] [CrossRef]
- Friedemann, M.; Kalbitzer, L.; Franz, S.; Moeller, S.; Schnabelrauch, M.; Simon, J.-C.; Pompe, T.; Franke, K. Instructing Human Macrophage Polarization by Stiffness and Glycosaminoglycan Functionalization in 3D Collagen Networks. Adv. Health Mater. 2017, 6, 1600967. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Lyu, C.; Zhao, P.; Li, W.; Kong, W.; Huang, C.; Genin, G.M.; Du, Y. Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D. Nat. Commun. 2019, 10, 3491. [Google Scholar] [CrossRef] [PubMed]
- Ghrebi, S.; Hamilton, D.W.; Waterfield, J.D.; Brunette, D.M. The effect of surface topography on cell shape and early ERK1/2 signaling in macrophages; linkage with FAK and Src. J. Biomed. Mater. Res. Part A 2013, 101, 2118–2128. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [PubMed]
- Hamlet, S.; Alfarsi, M.; George, R.; Ivanovski, S. The effect of hydrophilic titanium surface modification on macrophage inflammatory cytokine gene expression. Clin. Oral Implant. Res. 2011, 23, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2015, 31, 425–434. [Google Scholar] [CrossRef]
- Luu, T.U.; Gott, S.C.; Woo, B.W.K.; Rao, M.P.; Liu, W.F. Micro- and Nanopatterned Topographical Cues for Regulating Macrophage Cell Shape and Phenotype. ACS Appl. Mater. Interfaces 2015, 7, 28665–28672. [Google Scholar] [CrossRef]
- Malheiro, V.; Lehner, F.; Dinca, V.; Hoffmann, P.; Maniura-Weber, K. Convex and concave micro-structured silicone controls the shape, but not the polarization state of human macrophages. Biomater. Sci. 2016, 4, 1562–1573. [Google Scholar] [CrossRef]
- Kharaziha, M.; Baidya, A.; Annabi, N. Rational Design of Immunomodulatory Hydrogels for Chronic Wound Healing. Adv. Mater. 2021, 33, e2100176. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, L.; Atallah, P.; Werner, C.; Freudenberg, U. StarPEG-Heparin Hydrogels to Protect and Sustainably Deliver IL-4. Adv. Health Mater. 2016, 5, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Bonito, V.; Smits, A.I.P.M.; Goor, O.J.G.M.; Ippel, B.; Driessen-Mol, A.; Münker, T.; Bosman, A.; Mes, T.; Dankers, P.; Bouten, C. Modulation of macrophage phenotype and protein secretion via heparin-IL-4 functionalized supramolecular elastomers. Acta Biomater. 2018, 71, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, H.; Zhang, N.; Hu, L.; Jing, W.; Pan, J. Interleukin-4-loaded hydrogel scaffold regulates macrophages polarization to promote bone mesenchymal stem cells osteogenic differentiation via TGF-β1/Smad pathway for repair of bone defect. Cell Prolif. 2020, 53, e12907. [Google Scholar] [CrossRef]
- Xuan, X.; Zhou, Y.; Chen, A.; Zheng, S.; An, Y.; He, H.; Huang, W.; Chen, Y.; Yang, Y.; Li, S.; et al. Silver crosslinked injectable bFGF-eluting supramolecular hydrogels speed up infected wound healing. J. Mater. Chem. B 2019, 8, 1359–1370. [Google Scholar] [CrossRef]
- Yu, J.R.; Varrey, P.; Liang, B.J.; Huang, H.-C.; Fisher, J.P. Liposomal SDF-1 Alpha Delivery in Nanocomposite Hydrogels Promotes Macrophage Phenotype Changes and Skin Tissue Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 5230–5241. [Google Scholar] [CrossRef]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef]
- Seta, M.; Haraźna, K.; Kasarełło, K.; Solarz-Keller, D.; Cudnoch-Jędrzejewska, A.; Witko, T.; Rajfur, Z.; Guzik, M. The Influence of Novel, Biocompatible, and Bioresorbable Poly(3-hydroxyoctanoate) Dressings on Wound Healing in Mice. Int. J. Mol. Sci. 2022, 23, 16159. [Google Scholar] [CrossRef]
- Wei, Q.; Zhao, Y.; Wei, Y.; Wang, Y.; Jin, Z.; Ma, G.; Jiang, Y.; Zhang, W.; Hu, Z. Facile preparation of polyphenol-crosslinked chitosan-based hydrogels for cutaneous wound repair. Int. J. Biol. Macromol. 2023, 228, 99–110. [Google Scholar] [CrossRef]
- Moura, L.I.; Dias, A.M.; Suesca, E.; Casadiegos, S.; Leal, E.C.; Fontanilla, M.R.; Carvalho, L.; de Sousa, H.C.; Carvalho, E. Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice. Biochim. Biophys. Acta 2014, 1842, 32–43. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Q.; Li, Y.; Song, W.; Chen, A.; Liu, J.; Xuan, X. Collagen sponge prolongs taurine release for improved wound healing through inflammation inhibition and proliferation stimulation. Ann. Transl. Med. 2021, 9, 1010. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ma, Y.; Bao, Y.; Liu, Z.; Chen, L.; Dai, F.; Li, Z. Electrospun PLGA/SF/artemisinin composite nanofibrous membranes for wound dressing. Int. J. Biol. Macromol. 2021, 183, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Li, H.; Yang, Y.; Hou, L. Anti-Inflammatory and Immunoregulatory Functions of Artemisinin and Its Derivatives. Mediat. Inflamm. 2015, 2015, 435713. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Liu, H.; Zhu, Y.; Xia, D.; Wang, S.; Gu, R.; Zhang, P.; Liu, Y.; Zhou, Y. Flufenamic Acid Inhibits Adipogenic Differentiation of Mesenchymal Stem Cells by Antagonizing the PI3K/AKT Signaling Pathway. Stem Cells Int. 2020, 2020, 1540905. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-L.; Wang, Z.-L.; Fan, Z.-X.; Wu, M.-J.; Zhang, Y.; Ding, W.; Huang, Y.-Z.; Xie, H.-Q. Human adipose-derived stem cell-loaded small intestinal submucosa as a bioactive wound dressing for the treatment of diabetic wounds in rats. Biomater. Adv. 2022, 136, 212793. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kong, B.; Zhu, Y.; Zhao, Y. Bioactive Fish Scale Scaffolds with MSCs-Loading for Skin Flap Regeneration. Adv. Sci. 2022, 9, e2201226. [Google Scholar] [CrossRef] [PubMed]
- Zomer, H.D.; Jeremias, T.D.S.; Ratner, B.; Trentin, A.G. Mesenchymal stromal cells from dermal and adipose tissues induce macrophage polarization to a pro-repair phenotype and improve skin wound healing. Cytotherapy 2020, 22, 247–260. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, H.; Yue, Z.; Yu, C.; Jiang, L.; Dong, X.; Yao, M.; Shi, M.; Liang, L.; Wan, Y.; et al. Zwitterionic Polysaccharide-Based Hydrogel Dressing as a Stem Cell Carrier to Accelerate Burn Wound Healing. Adv. Health Mater. 2022, e2202309. [Google Scholar] [CrossRef]
- Xiao, S.; Xiao, C.; Miao, Y.; Wang, J.; Chen, R.; Fan, Z.; Hu, Z. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res. Ther. 2021, 12, 255. [Google Scholar] [CrossRef]
- Li, J.; Yan, S.; Han, W.; Dong, Z.; Li, J.; Wu, Q.; Fu, X. Phospholipids-grafted PLLA electrospun micro/nanofibers immobilized with small extracellular vesicles from rat adipose mesenchymal stem cells promote wound healing in diabetic rats. Regen. Biomater. 2022, 9, rbac071. [Google Scholar] [CrossRef]
- da Anunciação, A.; Mess, A.; Orechio, D.; Aguiar, B.; Favaron, P.; Miglino, M. Extracellular matrix in epitheliochorial, endotheliochorial and haemochorial placentation and its potential application for regenerative medicine. Reprod. Domest. Anim. 2016, 52, 3–15. [Google Scholar] [CrossRef]
- Padhi, A.; Nain, A.S. ECM in Differentiation: A Review of Matrix Structure, Composition and Mechanical Properties. Ann. Biomed. Eng. 2020, 48, 1071–1089. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Ding, F.; Gong, L.; Gu, X. Extracellular Matrix Scaffolds for Tissue Engineering and Regenerative Medicine. Curr. Stem Cell Res. Ther. 2017, 12, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, A.; Liu, S.; Lv, M.; Chen, S. Research progress in decellularized extracellular matrix-derived hydrogels. Regen. Ther. 2021, 18, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Dziki, J.L.; Wang, D.S.; Pineda, C.; Sicari, B.M.; Rausch, T.; Badylak, S.F. Solubilized extracellular matrix bioscaffolds derived from diverse source tissues differentially influence macrophage phenotype. J. Biomed. Mater. Res. Part A 2016, 105, 138–147. [Google Scholar] [CrossRef]
- Meng, F.W.; Slivka, P.F.; Dearth, C.L.; Badylak, S.F. Solubilized extracellular matrix from brain and urinary bladder elicits distinct functional and phenotypic responses in macrophages. Biomaterials 2015, 46, 131–140. [Google Scholar] [CrossRef]
- Huleihel, L.; Dziki, J.L.; Bartolacci, J.G.; Rausch, T.; Scarritt, M.E.; Cramer, M.C.; Vorobyov, T.; LoPresti, S.T.; Swineheart, I.T.; White, L.J.; et al. Macrophage phenotype in response to ECM bioscaffolds. Semin. Immunol. 2017, 29, 2–13. [Google Scholar] [CrossRef]
- Frazao, L.P.; Vieira de Castro, J.; Nogueira-Silva, C.; Neves, N.M. Decellularized Human Chorion Membrane as a Novel Biomaterial for Tissue Regeneration. Biomolecules 2020, 10, 1208. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Chung, J.; Kim, S.; Park, J.; Lee, K.; Jung, Y. Enhanced Regeneration of Vascularized Adipose Tissue with Dual 3D-Printed Elastic Polymer/dECM Hydrogel Complex. Int. J. Mol. Sci. 2021, 22, 2886. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, Y.; Yang, C.; Liu, X.; Huangfu, Y.; Zhang, C.; Huang, P.; Dong, A.; Liu, J.; Liu, J.; et al. Bioinspired and Inflammation-Modulatory Glycopeptide Hydrogels for Radiation-Induced Chronic Skin Injury Repair. Adv. Health Mater. 2022, 12, e2201671. [Google Scholar] [CrossRef] [PubMed]
- Seif-Naraghi, S.B.; Salvatore, M.A.; Schup-Magoffin, P.J.; Hu, D.P.; Christman, K.L. Design and Characterization of an Injectable Pericardial Matrix Gel: A Potentially Autologous Scaffold for Cardiac Tissue Engineering. Tissue Eng. Part A 2010, 16, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- El Masry, M.S.; Chaffee, S.; Das Ghatak, P.; Mathew-Steiner, S.S.; Das, A.; Higuita-Castro, N.; Roy, S.; Anani, R.A.; Sen, C.K. Stabilized collagen matrix dressing improves wound macrophage function and epithelialization. FASEB J. 2019, 33, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, D.; Bertani, F.; Fusaro, L.; Clemente, N.; Carton, F.; Talmon, M.; Fresu, L.G.; Boccafoschi, F. Regenerative Potential of A Bovine ECM-Derived Hydrogel for Biomedical Applications. Biomolecules 2022, 12, 1222. [Google Scholar] [CrossRef]
- Yu, F.; Khan, A.U.R.; Zheng, H.; Li, X.; El-Newehy, M.; El-Hamshary, H.; Morsi, Y.; Li, J.; Wu, J.; Mo, X. A photocrosslinking antibacterial decellularized matrix hydrogel with nanofiber for cutaneous wound healing. Colloids Surf. B Biointerfaces 2022, 217, 112691. [Google Scholar] [CrossRef]
- Savitri, C.; Ha, S.S.; Liao, E.; Du, P.; Park, K. Extracellular matrices derived from different cell sources and their effect on macrophage behavior and wound healing. J. Mater. Chem. B 2020, 8, 9744–9755. [Google Scholar] [CrossRef]
- Li, T.; Sun, M.; Wu, S. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef]
- Cui, C.; Sun, S.; Wu, S.; Chen, S.; Ma, J.; Zhou, F. Electrospun chitosan nanofibers for wound healing application. Eng. Regen. 2021, 2, 82–90. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, M.; Li, Y.; Li, K.; Chen, S.; Zhao, W.; Wu, S.; Han, Y. Electrospun Chitosan–Polyvinyl Alcohol Nanofiber Dressings Loaded with Bioactive Ursolic Acid Promoting Diabetic Wound Healing. Nanomaterials 2022, 12, 2933. [Google Scholar] [CrossRef]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef]
- Azari, A.; Golchin, A.; Maymand, M.M.; Mansouri, F.; Ardeshirylajimi, A. Electrospun Polycaprolactone Nanofibers: Current Research and Applications in Biomedical Application. Adv. Pharm. Bull. 2022, 12, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nwabor, O.F.; Sukri, D.M.; Wunnoo, S.; Dumjun, K.; Lethongkam, S.; Kusolphat, P.; Hemtanon, N.; Klinprathum, K.; Sunghan, J.; et al. Poly (vinyl alcohol) copolymerized with xanthan gum/hypromellose/sodium carboxymethyl cellulose dermal dressings functionalized with biogenic nanostructured materials for antibacterial and wound healing application. Int. J. Biol. Macromol. 2022, 216, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, M.; Wang, X.; Zhang, M.; Wang, X.; Li, Y.; Cui, Z.; Chen, X.; Han, Y.; Zhao, W. Electrospun multifunctional nanofibrous mats loaded with bioactive anemoside B4 for accelerated wound healing in diabetic mice. Drug Deliv. 2022, 29, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Chen, S.; Ge, W.; Zhao, Y.; Xu, X.; Wang, S.; Zhang, J. Riclin-Capped Silver Nanoparticles as an Antibacterial and Anti-Inflammatory Wound Dressing. Int. J. Nanomed. 2022, 17, 2629–2641. [Google Scholar] [CrossRef]
- Shitole, A.A.; Raut, P.; Giram, P.; Rade, P.; Khandwekar, A.; Garnaik, B.; Sharma, N. Poly (vinylpyrrolidone)-iodine engineered poly (ε-caprolactone) nanofibers as potential wound dressing materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110731. [Google Scholar] [CrossRef]
- Fu, Y.; Guan, J.; Guo, S.; Guo, F.; Niu, X.; Liu, Q.; Zhang, C.; Nie, H.; Wang, Y. Human urine-derived stem cells in combination with polycaprolactone/gelatin nanofibrous membranes enhance wound healing by promoting angiogenesis. J. Transl. Med. 2014, 12, 274. [Google Scholar] [CrossRef]
- Cao, W.; Peng, S.; Yao, Y.; Xie, J.; Li, S.; Tu, C.; Gao, C. A nanofibrous membrane loaded with doxycycline and printed with conductive hydrogel strips promotes diabetic wound healing in vivo. Acta Biomater. 2022, 152, 60–73. [Google Scholar] [CrossRef]
- Lv, F.; Wang, J.; Xu, P.; Han, Y.; Ma, H.; Xu, H.; Chen, S.; Chang, J.; Ke, Q.; Liu, M.; et al. A conducive bioceramic/polymer composite biomaterial for diabetic wound healing. Acta Biomater. 2017, 60, 128–143. [Google Scholar] [CrossRef]
- Thangavel, P.; Kannan, R.; Ramachandran, B.; Moorthy, G.; Suguna, L.; Muthuvijayan, V. Development of reduced graphene oxide (rGO)-isabgol nanocomposite dressings for enhanced vascularization and accelerated wound healing in normal and diabetic rats. J. Colloid Interface Sci. 2018, 517, 251–264. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Kim, T.M. Mesenchymal Stem Cell-derived Extracellular Vesicles for Skin Wound Healing. Adv. Exp. Med. Biol. 2021, 1310, 495–507. [Google Scholar] [CrossRef]
- Jo, H.; Brito, S.; Kwak, B.; Park, S.; Lee, M.-G.; Bin, B.-H. Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation. Int. J. Mol. Sci. 2021, 22, 2410. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Go, J.; Lee, J.H.; Han, D.-W.; Hwang, D.; Lee, J. Transdermal treatment of the surgical and burned wound skin via phytochemical-capped gold nanoparticles. Colloids Surf. B Biointerfaces 2015, 135, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, K.; Monisha, P.; Srinivasan, M.; Swathi, D.; Raman, M.; Raj, G.D. Chlorhexidine-calcium phosphate nanoparticles—Polymer mixer based wound healing cream and their applications. Mater. Sci. Eng C Mater. Biol. Appl. 2016, 67, 516–521. [Google Scholar] [CrossRef]
- Kang, H.; Kim, S.; Wong, D.S.H.; Jung, H.J.; Lin, S.; Zou, K.; Li, R.; Li, G.; Dravid, V.P.; Bian, L. Remote Manipulation of Ligand Nano-Oscillations Regulates Adhesion and Polarization of Macrophages in Vivo. Nano Lett. 2017, 17, 6415–6427. [Google Scholar] [CrossRef]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Włodarczyk-Biegun, M.K.; del Campo, A.; Vázquez-Lasa, B.; Román, J.S. Development of bioactive catechol functionalized nanoparticles applicable for 3D bioprinting. Mater. Sci. Eng C Mater. Biol. Appl. 2021, 131, 112515. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, W.; Shan, J.; He, J.; Qian, H.; Chen, X.; Wang, X. Thermosensitive Hydrogel Loaded with Nickel–Copper Bimetallic Hollow Nanospheres with SOD and CAT Enzymatic-Like Activity Promotes Acute Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 50677–50691. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, Z.; Liu, Y.; Zhang, W.; Geng, L.; Ni, T. Incorporation of ROS-Responsive Substance P-Loaded Zeolite Imidazolate Framework-8 Nanoparticles into a Ca2+-Cross-Linked Alginate/Pectin Hydrogel for Wound Dressing Applications. Int. J. Nanomed. 2020, 15, 333–346. [Google Scholar] [CrossRef]
- Wang, X.; Coradin, T.; Hélary, C. Modulating inflammation in a cutaneous chronic wound model by IL-10 released from collagen–silica nanocomposites via gene delivery. Biomater. Sci. 2017, 6, 398–406. [Google Scholar] [CrossRef]
- Qian, Y.; Zheng, Y.; Jin, J.; Wu, X.; Xu, K.; Dai, M.; Niu, Q.; Zheng, H.; He, X.; Shen, J. Immunoregulation in Diabetic Wound Repair with a Photoenhanced Glycyrrhizic Acid Hydrogel Scaffold. Adv. Mater. 2022, 34, e2200521. [Google Scholar] [CrossRef]
- Tang, J.; Li, H.; Peng, H.; Zhang, Z.; Liu, C.; Cheng, Y.; Wang, K.; Yu, Z.; Lyu, Z.; Zhang, J.; et al. Pre-clinical evaluation of thermosensitive decellularized adipose tissue/platelet-rich plasma interpenetrating polymer network hydrogel for wound healing. Mater. Today Bio 2022, 17, 100498. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, X.; Hu, C.; Li, P.; Zhao, M.; Lu, J.; Xia, G. A fucoidan-gelatin wound dressing accelerates wound healing by enhancing antibacterial and anti-inflammatory activities. Int. J. Biol. Macromol. 2022, 223 Pt A, 36–48. [Google Scholar] [CrossRef]
- Sharma, S.; Madhyastha, H.; Kirwale, S.S.; Sakai, K.; Katakia, Y.T.; Majumder, S.; Roy, A. Dual antibacterial and anti-inflammatory efficacy of a chitosan-chondroitin sulfate-based in-situ forming wound dressing. Carbohydr. Polym. 2022, 298, 120126. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Luo, X.; Huang, Z.; Midgley, A.C.; Wang, B.; Liu, R.; Zhi, D.; Wei, T.; Zhou, X.; Qiao, M.; et al. Cobalt-mediated multi-functional dressings promote bacteria-infected wound healing. Acta Biomater. 2019, 86, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Dong, Y.; Xu, P.; Pan, Q.; Jia, K.; Jin, P.; Zhou, M.; Xu, Y.; Guo, R.; Cheng, B. A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 2022, 154, 212–230. [Google Scholar] [CrossRef] [PubMed]
| Scaffold | Tissue/Cell Source | Wound Healing Outcomes | Macrophage Behaviors | Ref. |
|---|---|---|---|---|
| sPCM | Equine pericardium | Accelerated wound closure; increased collagen deposition and maturation. | Polarized into M2 phenotype; elevated the levels of IL-10, Arginase-1 and VEGF; and reduced the expression of IL-1β and TNF-α. | [93] |
| Pericardium dECM hydrogel | Bovine pericardium | Enhanced wound healing. | Polarized into M2 phenotype; reduced the expression of CD80/CD86; and increased the expression of CD163 and CD206. | [94] |
| SIS + ADSCs | SIS | Enhanced wound angiogenesis, re-epithelization, and skin appendage regeneration. | Promoted macrophage infiltration at the early stage of wound healing; reduced macrophage infiltration at later stages of wound healing; and raised the M2:M1 macrophage ratio. | [75] |
| DTP4 hydrogel | Decellularized dermal tissue | Raised wound closure ratio; reduced inflammatory response; and promoted re-epithelization, hair follicles regeneration, and collagen maturation. | Shifted to the healing phase quickly. | [95] |
| hFDM; UMDM | Human lung fibroblasts; umbilical cord-blood MSCs | Thinner epidermal layer; significant recovery of skin appendage; better neovascularization; and higher recruitment of myofibroblasts. | A large number of M2 macrophage; most of CD206+ cells in the dermis region; and ECM Hydrogel reserved more CD206+ cells than the other ones. | [96] |
| Nanofibrous Composites | Materials Combined with Nanofibers | Biological Function | Ref. |
|---|---|---|---|
| CS-PVA-UA | UA | Reduced M1 macrophage polarization and restored M2 polarization; improved wound closure rate; promoted re-epithelization, revascularization, collagen deposition, and remodeling; and stimulated hair follicles regeneration. | [99] |
| sEVs@DSPE-PLLA | adipose MSC-derived sEVs | Inhibited the expression of IL-1β and TNF-α; upregulated the expression of IL-10, Arginase 1, and CD206; stimulated fibroblast proliferation; and promoted collagen deposition and angiogenesis. | [80] |
| CS-PVA-ANE | ANE | Suppressed LPS-stimulated M1 macrophage polarization; reduced ROS production; decreased the level of inflammatory cytokines; enhanced wound closure; accelerated wound angiogenesis; and promoted wound re-epithelization and collagen deposition. | [103] |
| Isab + rGO | rGO | Stimulated collagen synthesis, collagen crosslinking, and wound contraction; reduced re-epithelization time; and reduced the presentence of CD68 positive cells. | [109] |
| PFKU nanofibrous membrane | DOXH | Promoted the migration of endothelial cells; promoted macrophage polarization into the M2 phenotype; downregulated the level of ROS and inflammatory factors; Upregulated the ratio of M2 macrophage; and promoted collagen deposition, revascularization, and re-epithelization. | [107] |
| PVP-I/PCL-poly-L-lysine nanofibers | PVP-I | Reduced the level of pro-inflammatory cytokines (TNF-α and IL-1β); promoted cell adhesion; and antimicrobial property. | [105] |
| PCL/gelatin nanofibers | NAGEL | Promoted the adhesion, proliferation, and migration of endothelial cells and keratinocytes; induced angiogenesis, collagen deposition, and re-epithelization of wounds; inhibited the inflammatory reactions; and activated EMT and EndMT pathways. | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.; Xiao, Y.; Guo, Z.; Shi, Y.; Tan, Q.; Huang, Y.; Xie, H. Modulation of Macrophage Function by Bioactive Wound Dressings with an Emphasis on Extracellular Matrix-Based Scaffolds and Nanofibrous Composites. Pharmaceutics 2023, 15, 794. https://doi.org/10.3390/pharmaceutics15030794
He T, Xiao Y, Guo Z, Shi Y, Tan Q, Huang Y, Xie H. Modulation of Macrophage Function by Bioactive Wound Dressings with an Emphasis on Extracellular Matrix-Based Scaffolds and Nanofibrous Composites. Pharmaceutics. 2023; 15(3):794. https://doi.org/10.3390/pharmaceutics15030794
Chicago/Turabian StyleHe, Tao, Yuzhen Xiao, Zhijun Guo, Yifeng Shi, Qiuwen Tan, Yizhou Huang, and Huiqi Xie. 2023. "Modulation of Macrophage Function by Bioactive Wound Dressings with an Emphasis on Extracellular Matrix-Based Scaffolds and Nanofibrous Composites" Pharmaceutics 15, no. 3: 794. https://doi.org/10.3390/pharmaceutics15030794
APA StyleHe, T., Xiao, Y., Guo, Z., Shi, Y., Tan, Q., Huang, Y., & Xie, H. (2023). Modulation of Macrophage Function by Bioactive Wound Dressings with an Emphasis on Extracellular Matrix-Based Scaffolds and Nanofibrous Composites. Pharmaceutics, 15(3), 794. https://doi.org/10.3390/pharmaceutics15030794