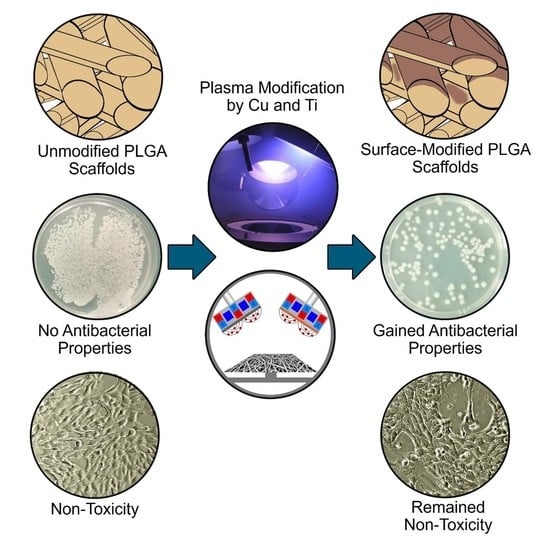
Antibacterial Activity and Cytocompatibility of Electrospun PLGA Scaffolds Surface-Modified by Pulsed DC Magnetron Co-Sputtering of Copper and Titanium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Poly(lactide-co-glycolide) (PLGA) Scaffolds
2.3. Surface Modification
2.4. Scanning Electron Microscopy
2.5. Porosity of the Scaffolds
2.6. Atomic Force Microscopy
2.7. Elemental and Chemical Analysis
2.8. Morphology and Elemental Mapping of Sample Cross-Sections
2.9. Mechanical Properties
2.10. Wettability
2.11. Thermal Gravimetric Analysis
2.12. Copper Ions Release
2.13. Morphology and Elemental Mapping of the Sample Surfaces
2.14. Antimicrobial Activity
2.15. Cytotoxicity towards NIH/3T3 Cells
2.16. Cytotoxicity towards Human Gingival Fibroblasts
2.17. Statistics
3. Results and Discussion
3.1. Morphology of the PLGA Scaffold Surfaces
3.2. Elemental and Chemical Composition of the PLGA Scaffold Surfaces
3.3. Morphology and Elemental Mapping of Sample Cross-Sections
3.4. Wettability and Mechanical Properties
- (1)
- Preservation of the surface morphology and surface roughness of PLGA scaffolds also after their Cu-Ti modification, which are reflected in the results of SEM and AFM (Figure 2).
- (2)
- Copper thin films, which are deposited on polymer substrates by magnetron sputtering, have a hydrophobic nature [78,79]. However, titanium thin films impart hydrophilic properties to polymer surfaces [80]. Thus, it can be concluded that even in the presence of copper on the surface of Cu-Ti-modified PLGA scaffolds in relatively low concentrations (Supplementary Material Figures S2b and S3b) is sufficient to preserve the hydrophobic properties.
- (3)
- Organic contaminations that are deposited on the modified scaffolds by a chemically active surface, adversely affect the wetting properties [81].
3.5. Thermal Gravimetric Analysis (TGA)
3.6. Copper Ion Release, Elemental Mapping, Antibacterial Activity
3.7. Toxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and Novel Polymeric Biomaterials for Neural Tissue Engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, H.; Zhang, K.; Kai, D.; Li, Z.; Loh, X.J. Polyester Elastomers for Soft Tissue Engineering. Chem. Soc. Rev. 2018, 47, 4545–4580. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun Nanofibers of Natural and Synthetic Polymers as Artificial Extracellular Matrix for Tissue Engineering. Nanomaterials 2020, 11, 21. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Perez-Puyana, V.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Influence of Elastin on the Properties of Hybrid <scp>PCL</Scp> /Elastin Scaffolds for Tissue Engineering. J. Appl. Polym. Sci. 2021, 138, 50893. [Google Scholar] [CrossRef]
- Vyas, C.; Zhang, J.; Øvrebø, Ø.; Huang, B.; Roberts, I.; Setty, M.; Allardyce, B.; Haugen, H.; Rajkhowa, R.; Bartolo, P. 3D Printing of Silk Microparticle Reinforced Polycaprolactone Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2021, 118, 111433. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D Printing of PLA/n-HA Composite Scaffolds with Customized Mechanical Properties and Biological Functions for Bone Tissue Engineering. Compos. Part B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Singh, D.; Babbar, A.; Jain, V.; Gupta, D.; Saxena, S.; Dwibedi, V. Synthesis, Characterization, and Bioactivity Investigation of Biomimetic Biodegradable PLA Scaffold Fabricated by Fused Filament Fabrication Process. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 121. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Gu, L.; Shan, T.; Ma, Y.; Tay, F.R.; Niu, L. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.C.; Kuo, S.-M.; Kong, A.M.; Morrison, W.A.; Dusting, G.J.; Mitchell, G.M.; Lim, S.Y.; Liu, G.-S. Three Dimensional Collagen Scaffold Promotes Intrinsic Vascularisation for Tissue Engineering Applications. PLoS ONE 2016, 11, e0149799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishan, A.P.; Nezarati, R.M.; Radzicki, C.M.; Renfro, A.L.; Robinson, J.L.; Whitely, M.E.; Cosgriff-Hernandez, E.M. In Situ Crosslinking of Electrospun Gelatin for Improved Fiber Morphology Retention and Tunable Degradation. J. Mater. Chem. B 2015, 3, 7930–7938. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Lee, C.H.; Cho, I.H.; Kim, Y.-J.; Lee, Y.-J.; Kim, I.A.; Park, K.-D.; Yui, N.; Shin, J.-W. Electrospun PLGA Nanofiber Scaffolds for Articular Cartilage Reconstruction: Mechanical Stability, Degradation and Cellular Responses under Mechanical Stimulation in Vitro. J. Biomater. Sci. Polym. Ed. 2006, 17, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, J.; Jin, K.; Liu, W.; Qiu, X.; Li, C. Fabrication of Functional PLGA-Based Electrospun Scaffolds and Their Applications in Biomedical Engineering. Mater. Sci. Eng. C 2016, 59, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, H.N.; Lee, S.J.; Song, J.E.; Kwon, S.Y.; Chung, J.W.; Lee, D.; Khang, G. Effect of Pore Sizes of PLGA Scaffolds on Mechanical Properties and Cell Behaviour for Nucleus Pulposus Regeneration in Vivo. J. Tissue Eng. Regen. Med. 2017, 11, 44–57. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D Electrospinning Technologies for the Fabrication of Nanofibrous Scaffolds for Skin Tissue Engineering: A Review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2020, 12, e1626. [Google Scholar] [CrossRef] [Green Version]
- Unal, S.; Arslan, S.; Yilmaz, B.K.; Oktar, F.N.; Ficai, D.; Ficai, A.; Gunduz, O. Polycaprolactone/Gelatin/Hyaluronic Acid Electrospun Scaffolds to Mimic Glioblastoma Extracellular Matrix. Materials 2020, 13, 2661. [Google Scholar] [CrossRef]
- Mombini, S.; Mohammadnejad, J.; Bakhshandeh, B.; Narmani, A.; Nourmohammadi, J.; Vahdat, S.; Zirak, S. Chitosan-PVA-CNT Nanofibers as Electrically Conductive Scaffolds for Cardiovascular Tissue Engineering. Int. J. Biol. Macromol. 2019, 140, 278–287. [Google Scholar] [CrossRef]
- He, P.; Zhong, Q.; Ge, Y.; Guo, Z.; Tian, J.; Zhou, Y.; Ding, S.; Li, H.; Zhou, C. Dual Drug Loaded Coaxial Electrospun PLGA/PVP Fiber for Guided Tissue Regeneration under Control of Infection. Mater. Sci. Eng. C 2018, 90, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent Advances in PLGA-Based Biomaterials for Bone Tissue Regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Han, F.; Zhang, W.; Yang, Y.; You, D.; Li, L. Toward Improved Wound Dressings: Effects of Polydopamine-Decorated Poly(Lactic- Co -Glycolic Acid) Electrospinning Incorporating Basic Fibroblast Growth Factor and Ponericin G1. RSC Adv. 2019, 9, 33038–33051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall Barrientos, I.J.; Paladino, E.; Szabó, P.; Brozio, S.; Hall, P.J.; Oseghale, C.I.; Passarelli, M.K.; Moug, S.J.; Black, R.A.; Wilson, C.G.; et al. Electrospun Collagen-Based Nanofibres: A Sustainable Material for Improved Antibiotic Utilisation in Tissue Engineering Applications. Int. J. Pharm. 2017, 531, 67–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzikowski, M.; Castanié, N.; Guedon, A.; Verrier, B.; Primard, C.; Sohier, J. Antibiotic Incorporation in Jet-Sprayed Nanofibrillar Biodegradable Scaffolds for Wound Healing. Int. J. Pharm. 2017, 532, 802–812. [Google Scholar] [CrossRef]
- Porter, M.L.A.; Münchow, E.A.; Albuquerque, M.T.P.; Spolnik, K.J.; Hara, A.T.; Bottino, M.C. Effects of Novel 3-Dimensional Antibiotic-Containing Electrospun Scaffolds on Dentin Discoloration. J. Endod. 2016, 42, 106–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Chellat, M.F.; Raguž, L.; Riedl, R. Targeting Antibiotic Resistance. Angew. Chemie Int. Ed. 2016, 55, 6600–6626. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic Resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Alavi, M.; Rai, M. Recent Advances in Antibacterial Applications of Metal Nanoparticles (MNPs) and Metal Nanocomposites (MNCs) against Multidrug-Resistant (MDR) Bacteria. Expert Rev. Anti. Infect. Ther. 2019, 17, 419–428. [Google Scholar] [CrossRef]
- Dai, X.; Guo, Q.; Zhao, Y.; Zhang, P.; Zhang, T.; Zhang, X.; Li, C. Functional Silver Nanoparticle as a Benign Antimicrobial Agent That Eradicates Antibiotic-Resistant Bacteria and Promotes Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 25798–25807. [Google Scholar] [CrossRef] [PubMed]
- Baig, U.; Ansari, M.A.; Gondal, M.A.; Akhtar, S.; Khan, F.A.; Falath, W.S. Single Step Production of High-Purity Copper Oxide-Titanium Dioxide Nanocomposites and Their Effective Antibacterial and Anti-Biofilm Activity against Drug-Resistant Bacteria. Mater. Sci. Eng. C 2020, 113, 110992. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Joo, S.H.; Toborek, M. Treatment of Antibiotic-Resistant Bacteria by Encapsulation of ZnO Nanoparticles in an Alginate Biopolymer: Insights into Treatment Mechanisms. J. Hazard. Mater. 2019, 373, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-Polymer Nanocomposites: An Excellent and Cost-Effective Biocide for Use on Antibacterial Surfaces. Mater. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Bajpai, M.; Sharma, L. Copper Nanoparticles Loaded Alginate-Impregnated Cotton Fabric with Antibacterial Properties. J. Appl. Polym. Sci. 2012, 126, E319–E326. [Google Scholar] [CrossRef]
- Zakharova, O.V.; Godymchuk, A.Y.; Gusev, A.A.; Gulchenko, S.I.; Vasyukova, I.A.; Kuznetsov, D.V. Considerable Variation of Antibacterial Activity of Cu Nanoparticles Suspensions Depending on the Storage Time, Dispersive Medium, and Particle Sizes. Biomed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Mungkalasiri, J.; Bedel, L.; Emieux, F.; Cara, A.V.-D.; Freney, J.; Maury, F.; Renaud, F.N.R. Antibacterial Properties of TiO2–Cu Composite Thin Films Grown by a One Step DLICVD Process. Surf. Coatings Technol. 2014, 242, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Mu, L.; Rutkowski, S.; Gai, M.; Frueh, J. Alginate Microparticle Arrays as Self-Polishing Bio-Fouling Release Coatings. J. Nanosci. Nanotechnol. 2019, 19, 8052–8062. [Google Scholar] [CrossRef]
- Mu, L.; Rutkowski, S.; Gai, M.; Tverdokhlebov, S.I.; Frueh, J. Copper Alginate Surface for Perpetual Self-Polishing and Anti-Biofouling Compound Release. Appl. Surf. Sci. 2021, 569, 151087. [Google Scholar] [CrossRef]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Toxicity of Metal Compounds: Knowledge and Myths. Organometallics 2017, 36, 4071–4090. [Google Scholar] [CrossRef] [Green Version]
- Moniri Javadhesari, S.; Alipour, S.; Akbarpour, M.R. Biocompatibility, Osseointegration, Antibacterial and Mechanical Properties of Nanocrystalline Ti-Cu Alloy as a New Orthopedic Material. Colloids Surf. B Biointerfaces 2020, 189, 110889. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, H.; Wang, X.; Yang, L.; Chen, M.; Wang, R.; Qin, G.; Chen, D.-F.; Zhang, E. Effect of Ultrasonic Micro-Arc Oxidation on the Antibacterial Properties and Cell Biocompatibility of Ti-Cu Alloy for Biomedical Application. Mater. Sci. Eng. C 2020, 115, 110921. [Google Scholar] [CrossRef] [PubMed]
- Kylián, O.; Shelemin, A.; Solař, P.; Pleskunov, P.; Nikitin, D.; Kuzminova, A.; Štefaníková, R.; Kúš, P.; Cieslar, M.; Hanuš, J.; et al. Magnetron Sputtering of Polymeric Targets: From Thin Films to Heterogeneous Metal/Plasma Polymer Nanoparticles. Materials 2019, 12, 2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khamseh, S.; Alibakhshi, E.; Mahdavian, M.; Saeb, M.R.; Vahabi, H.; Kokanyan, N.; Laheurte, P. Magnetron-Sputtered Copper/Diamond-like Carbon Composite Thin Films with Super Anti-Corrosion Properties. Surf. Coatings Technol. 2018, 333, 148–157. [Google Scholar] [CrossRef]
- Adamiak, B.; Wiatrowski, A.; Domaradzki, J.; Kaczmarek, D.; Wojcieszak, D.; Mazur, M. Preparation of Multicomponent Thin Films by Magnetron Co-Sputtering Method: The Cu-Ti Case Study. Vacuum 2019, 161, 419–428. [Google Scholar] [CrossRef]
- Badaraev, A.D.; Koniaeva, A.; Krikova, S.A.; Shesterikov, E.V.; Bolbasov, E.N.; Nemoykina, A.L.; Bouznik, V.M.; Stankevich, K.S.; Zhukov, Y.M.; Mishin, I.P.; et al. Piezoelectric Polymer Membranes with Thin Antibacterial Coating for the Regeneration of Oral Mucosa. Appl. Surf. Sci. 2020, 504, 144068. [Google Scholar] [CrossRef]
- Sidelev, D.V.; Bleykher, G.A.; Bestetti, M.; Krivobokov, V.P.; Vicenzo, A.; Franz, S.; Brunella, M.F. A Comparative Study on the Properties of Chromium Coatings Deposited by Magnetron Sputtering with Hot and Cooled Target. Vacuum 2017, 143, 479–485. [Google Scholar] [CrossRef]
- Badaraev, A.D.; Sidelev, D.V.; Yurjev, Y.N.; Bukal, V.R.; Tverdokhlebov, S.I. Modes Development of PLGA Scaffolds Modification by Magnetron Co-Sputtering of Cu and Ti Targets. J. Phys. Conf. Ser. 2021, 1799, 012001. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Kemme, M.; Heinzel-Wieland, R. Quantitative Assessment of Antimicrobial Activity of PLGA Films Loaded with 4-Hexylresorcinol. J. Funct. Biomater. 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yao, Y.; Sullivan, N.; Chen, Y. Modeling the Primary Size Effects of Citrate-Coated Silver Nanoparticles on Their Ion Release Kinetics. Environ. Sci. Technol. 2011, 45, 4422–4428. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Ma, D.; Li, Y.; Jing, P.; Huang, B.; Jing, F.; Xie, D.; Leng, Y.; Akhavan, B.; Huang, N. Ti–Cu Coatings Deposited by a Combination of HiPIMS and DC Magnetron Sputtering: The Role of Vacuum Annealing on Cu Diffusion, Microstructure, and Corrosion Resistance. Coatings 2020, 10, 1064. [Google Scholar] [CrossRef]
- Palza, H.; Quijada, R.; Delgado, K. Antimicrobial Polymer Composites with Copper Micro- and Nanoparticles: Effect of Particle Size and Polymer Matrix. J. Bioact. Compat. Polym. 2015, 30, 366–380. [Google Scholar] [CrossRef]
- Villapún, V.M.; Esat, F.; Bull, S.; Dover, L.G.; González, S. Tuning the Mechanical and Antimicrobial Performance of a Cu-Based Metallic Glass Composite through Cooling Rate Control and Annealing. Materials 2017, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Wang, N.; Chen, Y.; He, B.; Zhao, Q.; Chen, L.; Tang, Y.; Luo, B.; Zhao, Y.; Yang, X. Biological Corrosion Behaviour and Antibacterial Properties of Ti-Cu Alloy with Different Ti2Cu Morphologies for Dental Applications. Mater. Des. 2022, 215, 110540. [Google Scholar] [CrossRef]
- Mejía, V.H.D.; Echavarría, A.M.; Calderón, J.A.; Bejarano, G.G. Microstructural and Electrochemical Properties of TiAlN(Ag,Cu) Nanocomposite Coatings for Medical Applications Deposited by Dc Magnetron Sputtering. J. Alloys Compd. 2020, 828, 154396. [Google Scholar] [CrossRef]
- Yu, C.-C.; Chen, Y.-W.; Yeh, P.-Y.; Hsiao, Y.-S.; Lin, W.-T.; Kuo, C.-W.; Chueh, D.-Y.; You, Y.-W.; Shyue, J.-J.; Chang, Y.-C.; et al. Random and Aligned Electrospun PLGA Nanofibers Embedded in Microfluidic Chips for Cancer Cell Isolation and Integration with Air Foam Technology for Cell Release. J. Nanobiotechnology 2019, 17, 31. [Google Scholar] [CrossRef] [Green Version]
- Purusottam Reddy, B.; Sivajee Ganesh, K.; Hussain, O.M. Growth, Microstructure and Supercapacitive Performance of Copper Oxide Thin Films Prepared by RF Magnetron Sputtering. Appl. Phys. A 2016, 122, 128. [Google Scholar] [CrossRef]
- Mazur, M.; Domaradzki, J.; Wojcieszak, D.; Kaczmarek, D. Investigations of Elemental Composition and Structure Evolution in (Ti,Cu)-Oxide Gradient Thin Films Prepared Using (Multi)Magnetron Co-Sputtering. Surf. Coatings Technol. 2018, 334, 150–157. [Google Scholar] [CrossRef]
- Yang, F.; Hu, G.; He, H.; Yi, M.; Ge, Y.; Ran, L.; Peng, K. Effect of Amorphous Carbon on the Tensile Behavior of Polyacrylonitrile (PAN)-Based Carbon Fibers. J. Mater. Sci. 2019, 54, 8800–8813. [Google Scholar] [CrossRef]
- Badaraev, A.D.; Sidelev, D.V.; Kozelskaya, A.I.; Bolbasov, E.N.; Tran, T.-H.; Nashchekin, A.V.; Malashicheva, A.B.; Rutkowski, S.; Tverdokhlebov, S.I. Surface Modification of Electrospun Bioresorbable and Biostable Scaffolds by Pulsed DC Magnetron Sputtering of Titanium for Gingival Tissue Regeneration. Polymers 2022, 14, 4922. [Google Scholar] [CrossRef] [PubMed]
- Matikainen, A.; Nuutinen, T.; Itkonen, T.; Heinilehto, S.; Puustinen, J.; Hiltunen, J.; Lappalainen, J.; Karioja, P.; Vahimaa, P. Atmospheric Oxidation and Carbon Contamination of Silver and Its Effect on Surface-Enhanced Raman Spectroscopy (SERS). Sci. Rep. 2016, 6, 37192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajendiran, M.; Jainuddin Yousuf, S.M.; Elangovan, V.; Balasubramanian, S. Gold Nanoparticle Conjugated PLGA–PEG–SA–PEG–PLGA Multiblock Copolymer Nanoparticles: Synthesis, Characterization, in Vivo Release of Rifampicin. J. Mater. Chem. B 2014, 2, 418–427. [Google Scholar] [CrossRef]
- Spek, S.; Haeuser, M.; Schaefer, M.M.; Langer, K. Characterisation of PEGylated PLGA Nanoparticles Comparing the Nanoparticle Bulk to the Particle Surface Using UV/Vis Spectroscopy, SEC, 1H NMR Spectroscopy, and X-Ray Photoelectron Spectroscopy. Appl. Surf. Sci. 2015, 347, 378–385. [Google Scholar] [CrossRef]
- Esmaili, Z.; Bayrami, S.; Dorkoosh, F.A.; Akbari Javar, H.; Seyedjafari, E.; Zargarian, S.S.; Haddadi-Asl, V. Development and Characterization of Electrosprayed Nanoparticles for Encapsulation of Curcumin. J. Biomed. Mater. Res. Part A 2018, 106, 285–292. [Google Scholar] [CrossRef]
- Schoeller, J.; Itel, F.; Wuertz-Kozak, K.; Gaiser, S.; Luisier, N.; Hegemann, D.; Ferguson, S.J.; Fortunato, G.; Rossi, R.M. PH-Responsive Chitosan/Alginate Polyelectrolyte Complexes on Electrospun PLGA Nanofibers for Controlled Drug Release. Nanomaterials 2021, 11, 1850. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. The Same Chemical State of Carbon Gives Rise to Two Peaks in X-Ray Photoelectron Spectroscopy. Sci. Rep. 2021, 11, 11195. [Google Scholar] [CrossRef]
- Irfan, M.; Perero, S.; Miola, M.; Maina, G.; Ferri, A.; Ferraris, M.; Balagna, C. Antimicrobial Functionalization of Cotton Fabric with Silver Nanoclusters/Silica Composite Coating via RF Co-Sputtering Technique. Cellulose 2017, 24, 2331–2345. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Xie, J.; Xue, L. Calibration of Binding Energy Positions with C1s for XPS Results. J. Wuhan Univ. Technol. Sci. Ed. 2020, 35, 711–718. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced Analysis of Copper X-Ray Photoelectron Spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Gonbeau, D.; Guimon, C.; Pfister-Guillouzo, G.; Levasseur, A.; Meunier, G.; Dormoy, R. XPS Study of Thin Films of Titanium Oxysulfides. Surf. Sci. 1991, 254, 81–89. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, M.; Yang, Y.; Zhang, Y.; Yan, F.; Li, H. Excellent Mechanical, Tribological and Anti-Corrosive Performance of Novel Ti-DLC Nanocomposite Thin Films Prepared via Magnetron Sputtering Method. Carbon N. Y. 2019, 151, 136–147. [Google Scholar] [CrossRef]
- Achour, A.; Islam, M.; Solaymani, S.; Vizireanu, S.; Saeed, K.; Dinescu, G. Influence of Plasma Functionalization Treatment and Gold Nanoparticles on Surface Chemistry and Wettability of Reactive-Sputtered TiO2 Thin Films. Appl. Surf. Sci. 2018, 458, 678–685. [Google Scholar] [CrossRef]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef]
- Platzman, I.; Brener, R. Oxidation of Polycrystalline Copper Thin Films at Ambient Conditions. J. Phys. Chem. C 2008, 112, 1101–1108. [Google Scholar] [CrossRef]
- Wucher, A.; Reuter, W. Angular Distribution of Particles Sputtered from Metals and Alloys. J. Vac. Sci. Technol. A Vac. Surf. Film. 1988, 6, 2316–2318. [Google Scholar] [CrossRef]
- Peng, L.; Guo, R.; Lan, J.; Jiang, S.; Zhang, Z.; Xu, J. Preparation and Characterization of Copper-Coated Polyester Fabric Pretreated with Laser by Magnetron Sputtering. J. Ind. Text. 2018, 48, 482–493. [Google Scholar] [CrossRef]
- Huang, M.-L.; Cai, Z.; Wu, Y.-Z.; Lu, S.-G.; Luo, B.-S.; Li, Y.-H. Metallic Coloration on Polyester Fabric with Sputtered Copper and Copper Oxides Films. Vacuum 2020, 178, 109489. [Google Scholar] [CrossRef]
- Bolbasov, E.N.; Maryin, P.V.; Stankevich, K.S.; Kozelskaya, A.I.; Shesterikov, E.V.; Khodyrevskaya, Y.I.; Nasonova, M.V.; Shishkova, D.K.; Kudryavtseva, Y.A.; Anissimov, Y.G.; et al. Surface Modification of Electrospun Poly-(l-Lactic) Acid Scaffolds by Reactive Magnetron Sputtering. Colloids Surf. B Biointerfaces 2018, 162, 43–51. [Google Scholar] [CrossRef]
- Forrest, E.; Schulze, R.; Liu, C.; Dombrowski, D. Influence of Surface Contamination on the Wettability of Heat Transfer Surfaces. Int. J. Heat Mass Transf. 2015, 91, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Wojcieszak, D.; Osekowska, M.; Kaczmarek, D.; Szponar, B.; Mazur, M.; Mazur, P.; Obstarczyk, A. Influence of Material Composition on Structure, Surface Properties and Biological Activity of Nanocrystalline Coatings Based on Cu and Ti. Coatings 2020, 10, 343. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhang, Z.; Zhang, J.; Qin, G.; Zhang, E. Construction of a TiO2/Cu2O Multifunctional Coating on Ti-Cu Alloy and Its Influence on the Cell Compatibility and Antibacterial Properties. Surf. Coatings Technol. 2021, 421, 127438. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram-Positive Bacterial Infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of Antibacterial Activity of Copper Nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Huang, C.-F.; Ou, K.-L.; Peng, P.-W. Mechanical Properties and Antibacterial Activity of Copper Doped Diamond-like Carbon Films. Surf. Coatings Technol. 2011, 206, 1037–1040. [Google Scholar] [CrossRef]
- Sadeghi-Kiakhani, M.; Khamseh, S.; Rafie, A.; Tekieh, S.M.F.; Zarrintaj, P.; Saeb, M.R. Thermally Stable Antibacterial Wool Fabrics Surface-Decorated by TiON and TiON/Cu Thin Films. Surf. Innov. 2018, 6, 258–265. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, E.; Yang, K. Microstructure, Corrosion Properties and Bio-Compatibility of Calcium Zinc Phosphate Coating on Pure Iron for Biomedical Application. Mater. Sci. Eng. C 2014, 34, 201–206. [Google Scholar] [CrossRef]
- Huang, B.; Jing, F.; Akhavan, B.; Ji, L.; Leng, Y.; Xie, D.; Bilek, M.; Huang, N. Multifunctional Ti-XCu Coatings for Cardiovascular Interfaces: Control of Microstructure and Surface Chemistry. Mater. Sci. Eng. C 2019, 104, 109969. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badaraev, A.D.; Lerner, M.I.; Bakina, O.V.; Sidelev, D.V.; Tran, T.-H.; Krinitcyn, M.G.; Malashicheva, A.B.; Cherempey, E.G.; Slepchenko, G.B.; Kozelskaya, A.I.; et al. Antibacterial Activity and Cytocompatibility of Electrospun PLGA Scaffolds Surface-Modified by Pulsed DC Magnetron Co-Sputtering of Copper and Titanium. Pharmaceutics 2023, 15, 939. https://doi.org/10.3390/pharmaceutics15030939
Badaraev AD, Lerner MI, Bakina OV, Sidelev DV, Tran T-H, Krinitcyn MG, Malashicheva AB, Cherempey EG, Slepchenko GB, Kozelskaya AI, et al. Antibacterial Activity and Cytocompatibility of Electrospun PLGA Scaffolds Surface-Modified by Pulsed DC Magnetron Co-Sputtering of Copper and Titanium. Pharmaceutics. 2023; 15(3):939. https://doi.org/10.3390/pharmaceutics15030939
Chicago/Turabian StyleBadaraev, Arsalan D., Marat I. Lerner, Olga V. Bakina, Dmitrii V. Sidelev, Tuan-Hoang Tran, Maksim G. Krinitcyn, Anna B. Malashicheva, Elena G. Cherempey, Galina B. Slepchenko, Anna I. Kozelskaya, and et al. 2023. "Antibacterial Activity and Cytocompatibility of Electrospun PLGA Scaffolds Surface-Modified by Pulsed DC Magnetron Co-Sputtering of Copper and Titanium" Pharmaceutics 15, no. 3: 939. https://doi.org/10.3390/pharmaceutics15030939
APA StyleBadaraev, A. D., Lerner, M. I., Bakina, O. V., Sidelev, D. V., Tran, T. -H., Krinitcyn, M. G., Malashicheva, A. B., Cherempey, E. G., Slepchenko, G. B., Kozelskaya, A. I., Rutkowski, S., & Tverdokhlebov, S. I. (2023). Antibacterial Activity and Cytocompatibility of Electrospun PLGA Scaffolds Surface-Modified by Pulsed DC Magnetron Co-Sputtering of Copper and Titanium. Pharmaceutics, 15(3), 939. https://doi.org/10.3390/pharmaceutics15030939