Therapeutic Agent-Loaded Fibrous Scaffolds for Biomedical Applications
Abstract
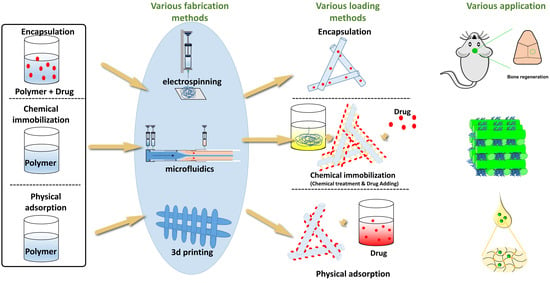
:1. Introduction
2. Natural Materials for Fibrous Scaffolds
2.1. Polysaccharides
2.1.1. Alginate
2.1.2. Chitosan
2.1.3. Hyaluronic Acid
2.2. Proteins
2.2.1. Collagen
2.2.2. Silk Fibroin
2.2.3. Elastin

3. Fabrication Methods
3.1. Electrospinning
3.1.1. Bicomponent Spinning
3.1.2. Coaxial Electrospinning
3.1.3. Multijet Electrospinning
3.1.4. Emulsion Electrospinning
3.2. Three-Dimensional Printing
3.2.1. Fused Deposition Modeling
3.2.2. Stereolithography
3.2.3. Selective Laser Sintering
3.2.4. Selective Laser Melting
3.3. Microfluidics
3.3.1. Principle of the Microfluidic Synthesis of Fiber
3.3.2. Microfluidic Devices
3.4. Solution Blowing
3.5. Centrifugal Spinning
4. Drug-Loading Methods
4.1. Blending
4.2. Chemical Immobilization
4.3. Physical Adsorption
4.4. Emulsion Electrospinning
4.5. Coaxial Electrospinning
4.6. Electrospray Technology
5. Biomedical Applications of the Therapeutic Agent-Loaded Fibrous Scaffolds
5.1. Scaffolds for Cardiovascular Regeneration and Vascularization
5.2. Regenerating Agent-Loaded Fibrous Scaffolds for Nerve Regeneration
5.3. Bone Regeneration
5.4. Scaffolds for Wound Healing
5.5. Anticancer Drug-Loaded Fibrous Scaffolds for Inhibiting the Recurrence and Metastasis of Cancer
5.6. Additional Therapeutic Applications of the Fibrous Scaffolds
6. Future Perspective and Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Jin, S.; Luo, D.; He, D.; Shi, C.; Zhu, L.; Guan, B.; Li, Z.; Zhang, T.; Zhou, Y.; et al. Functional Regeneration and Repair of Tendons Using Biomimetic Scaffolds Loaded with Recombinant Periostin. Nat. Commun. 2021, 12, 1293. [Google Scholar] [CrossRef]
- Varghese, J.; Rajagopal, A.; Shanmugasundaram, S. Role of Biomaterials Used for Periodontal Tissue Regeneration—A Concise Evidence-Based Review. Polymers 2022, 14, 3038. [Google Scholar] [CrossRef]
- Pattnaik, A.; Sanket, A.S.; Pradhan, S.; Sahoo, R.; Das, S.; Pany, S.; Douglas, T.E.L.; Dandela, R.; Liu, Q.; Rajadas, J.; et al. Designing of Gradient Scaffolds and Their Applications in Tissue Regeneration. Biomaterials 2023, 296, 122078. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Pham-Nguyen, O.V.; Yoo, H.S. Coaxial Electrospun Nanofibers with Different Shell Contents to Control Cell Adhesion and Viability. ACS Omega 2020, 5, 28178–28185. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, R.; Del Valle, L.J.; Torras, J.; Puiggalí, J. Recent Progress on Biodegradable Tissue Engineering Scaffolds Prepared by Thermally-Induced Phase Separation (Tips). Int. J. Mol. Sci. 2021, 22, 3504. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.Y.; Kim, I.S.; Zhang, K.Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical Electrospinning and 3D Printing Scaffold Design for Bone Regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural Polymers: Polysaccharides and Their Derivatives for Biomedical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780123969835. [Google Scholar]
- Gonçalves, R.C.; Banfi, A.; Oliveira, M.B.; Mano, J.F. Strategies for Re-Vascularization and Promotion of Angiogenesis in Trauma and Disease. Biomaterials 2021, 269, 120628. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Jiang, W.; Zuo, W.; Han, G. Influence of Stage Cooling Method on Pore Architecture of Biomimetic Alginate Scaffolds. Sci. Rep. 2017, 7, 16150. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Chi, Y.; Wang, Y.; Jiang, L.; Xu, N.; Wu, Q.; Feng, Q.; Sun, X. Preparation of Oriented Collagen Fiber Scaffolds and Its Application in Bone Tissue Engineering. Appl. Mater. Today 2021, 22, 100902. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Comprehensive Review on Electrospinning Techniques as Versatile Approaches toward Antimicrobial Biopolymeric Composite Fibers. Mater. Sci. Eng. C 2019, 101, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.W.; Cabodi, M.; Held, B.; Gleghorn, J.P.; Bonassar, L.J.; Stroock, A.D. Microfluidic Scaffolds for Tissue Engineering. Nat. Mater. 2007, 6, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yu, X.; Yu, B.; Yang, X.; Fu, Z.; Wan, J.; Zhu, M.; Wang, X.; Lin, K. Coaxially Fabricated Dual-Drug Loading Electrospinning Fibrous Mat with Programmed Releasing Behavior to Boost Vascularized Bone Regeneration. Adv. Healthc. Mater. 2022, 11, 2200571. [Google Scholar] [CrossRef]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M.; Vadivelu, J. A Review of Natural Polysaccharides for Drug Delivery Applications: Special Focus on Cellulose, Starch and Glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Urbanova, M.; Pavelkova, M.; Czernek, J.; Kubova, K.; Vyslouzil, J.; Pechova, A.; Molinkova, D.; Vyslouzil, J.; Vetchy, D.; Brus, J. Interaction Pathways and Structure-Chemical Transformations of Alginate Gels in Physiological Environments. Biomacromolecules 2019, 20, 4158–4170. [Google Scholar] [CrossRef]
- Novoa-Carballal, R.; Fernandez-Megia, E.; Riguera, R. Dynamics of Chitosan by 1H NMR Relaxation. Biomacromolecules 2010, 11, 2079–2086. [Google Scholar] [CrossRef]
- Levengood, S.K.L.; Zhang, M. Chitosan-Based Scaffolds for Bone Tissue Engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Rosellini, E.; Cascone, M.G. Microfluidic Fabrication of Natural Polymer-Based Scaffolds for Tissue Engineering Applications: A Review. Biomimetics 2023, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-Based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3D Bioprinting. J. Biol. Eng. 2020, 14, 8, Erratum in J. Biol. Eng. 2020, 14, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Novikova, L.N.; Mosahebi, A.; Wiberg, M.; Terenghi, G.; Kellerth, J.-O.; Novikov, L.N. Alginate Hydrogel and Matrigel as Potential Cell Carriers for Neurotransplantation. J. Biomed. Mater. Res. Part A 2006, 77, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan Based Bioactive Materials in Tissue Engineering Applications—A Review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef]
- Ji, C.; Annabi, N.; Khademhosseini, A.; Dehghani, F. Fabrication of Porous Chitosan Scaffolds for Soft Tissue Engineering Using Dense Gas CO2. Acta Biomater. 2011, 7, 1653–1664. [Google Scholar] [CrossRef]
- Lim, S.G.; Seo, S.E.; Park, S.J.; Kim, J.; Kim, Y.; Kim, K.H.; An, J.E.; Kwon, O.S. Real-Time Monitoring of Serotonin with Highly Selective Aptamer-Functionalized Conducting Polymer Nanohybrids. Nano Converg. 2022, 9, 31. [Google Scholar] [CrossRef]
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Determining the Deacetylation Degree of Chitosan: Opportunities To Learn Instrumental Techniques. J. Chem. Educ. 2018, 95, 1022–1028. [Google Scholar] [CrossRef]
- Al-Rooqi, M.M.; Hassan, M.M.; Moussa, Z.; Obaid, R.J.; Suman, N.H.; Wagner, M.H.; Natto, S.S.A.; Ahmed, S.A. Advancement of Chitin and Chitosan as Promising Biomaterials. J. Saudi Chem. Soc. 2022, 26, 101561. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Moseley, R.; Leaver, M.; Walker, M.; Waddington, R.J.; Parsons, D.; Chen, W.Y.J.; Embery, G. Comparison of the Antioxidant Properties of HYAFF®-11p75, AQUACEL® and Hyaluronan towards Reactive Oxygen Species In Vitro. Biomaterials 2002, 23, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In Vivo Bioresponses to Silk Proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef]
- Wang, X.; Kim, H.J.; Wong, C.; Vepari, C.; Matsumoto, A.; Kaplan, D.L. Fibrous Proteins and Tissue Engineering. Mater. Today 2006, 9, 44–53. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Arun, A.; Malrautu, P.; Laha, A.; Luo, H.; Ramakrishna, S. Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering. Appl. Sci. 2021, 11, 11369. [Google Scholar] [CrossRef]
- Elango, J.; Hou, C.; Bao, B.; Wang, S.; Maté Sánchez de Val, J.E.; Wenhui, W. The Molecular Interaction of Collagen with Cell Receptors for Biological Function. Polymers 2022, 14, 876. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen Extraction Process. Int. Food Res. J. 2016, 23, 913–922. [Google Scholar]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef]
- Liu, S.; Lau, C.-S.; Liang, K.; Wen, F.; Teoh, S.H. Marine Collagen Scaffolds in Tissue Engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef] [PubMed]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-Based Biomaterials for Biomedical Applications. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Buehler, M.J. Nature Designs Tough Collagen: Explaining the Nanostructure of Collagen Fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 12285–12290. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for Bone Tissue Regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Fang, G.; Sapru, S.; Behera, S.; Yao, J.; Shao, Z.; Kundu, S.C.; Chen, X. Exploration of the Tight Structural-Mechanical Relationship in Mulberry and Non-Mulberry Silkworm Silks. J. Mater. Chem. B 2016, 4, 4337–4347. [Google Scholar] [CrossRef]
- Dou, H.; Zuo, B. Effect of Sodium Carbonate Concentrations on the Degumming and Regeneration Process of Silk Fibroin. J. Text. Inst. 2015, 106, 311–319. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk Fibroin of Bombyx mori Is Secreted, Assembling a High Molecular Mass Elementary Unit Consisting of H-Chain, L-Chain, and P25, with a 6:6:1 Molar Ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef]
- Cebe, P.; Partlow, B.P.; Kaplan, D.L.; Wurm, A.; Zhuravlev, E.; Schick, C. Silk I and Silk II Studied by Fast Scanning Calorimetry. Acta Biomater. 2017, 55, 323–332. [Google Scholar] [CrossRef]
- Koh, L.D.; Cheng, Y.; Teng, C.P.; Khin, Y.W.; Loh, X.J.; Tee, S.Y.; Low, M.; Ye, E.; Yu, H.D.; Zhang, Y.W.; et al. Structures, Mechanical Properties and Applications of Silk Fibroin Materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Y.; Dai, W. Silk Fibroin-Based Biomaterials for Musculoskeletal Tissue Engineering. Mater. Sci. Eng. C 2018, 89, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Horan, R.L.; Lu, H.H.; Moreau, J.; Martin, I.; Richmond, J.C.; Kaplan, D.L. Silk Matrix for Tissue Engineered Anterior Cruciate Ligaments. Biomaterials 2002, 23, 4131–4141. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tao, W.; Lu, S.; Zhao, C. Porous 3-D Scaffolds from Regenerated Antheraea Pernyi Silk Fibroin. Polym. Adv. Technol. 2008, 19, 207–212. [Google Scholar] [CrossRef]
- Gregory, D.A.; Zhang, Y.; Smith, P.J.; Zhao, X.; Ebbens, S.J. Reactive Inkjet Printing of Biocompatible Enzyme Powered Silk Micro-Rockets. Small 2016, 12, 4048–4055. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk Fibroin as Biomaterial for Bone Tissue Engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Yang, B.-E.; Ahn, J.-H.; Park, S.O.; Shim, H.-W. Comparable Efficacy of Silk Fibroin with the Collagen Membranes for Guided Bone Regeneration in Rat Calvarial Defects. J. Adv. Prosthodont. 2014, 6, 539–546. [Google Scholar] [CrossRef]
- Meinel, L.; Hofmann, S.; Karageorgiou, V.; Kirker-Head, C.; McCool, J.; Gronowicz, G.; Zichner, L.; Langer, R.; Vunjak-Novakovic, G.; Kaplan, D.L. The Inflammatory Responses to Silk Films in Vitro and in Vivo. Biomaterials 2005, 26, 147–155. [Google Scholar] [CrossRef]
- Rastogi, S.; Kandasubramanian, B. Progressive Trends in Heavy Metal Ions and Dyes Adsorption Using Silk Fibroin Composites. Environ. Sci. Pollut. Res. 2020, 27, 210–237. [Google Scholar] [CrossRef]
- Reizabal, A.; Costa, C.M.; Pérez-Álvarez, L.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. Silk Fibroin as Sustainable Advanced Material: Material Properties and Characteristics, Processing, and Applications. Adv. Funct. Mater. 2023, 33, 2210764. [Google Scholar] [CrossRef]
- Murphy, A.R.; Kaplan, D.L. Biomedical Applications of Chemically-Modified Silk Fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef] [PubMed]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Drug and Gene Delivery. Pharmaceutics 2019, 11, 494. [Google Scholar] [CrossRef]
- Wongpinyochit, T.; Johnston, B.F.; Seib, F.P. Degradation Behavior of Silk Nanoparticles-Enzyme Responsiveness. ACS Biomater. Sci. Eng. 2018, 4, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Debelle, L.; Tamburro, A.M. Elastin: Molecular Description and Function. Int. J. Biochem. Cell Biol. 1999, 31, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Rock, M.J.; Cain, S.A.; Freeman, L.J.; Morgan, A.; Mellody, K.; Marson, A.; Shuttleworth, C.A.; Weiss, A.S.; Kielty, C.M. Molecular Basis of Elastic Fiber Formation: Critical interactions and a tropoelastin-fibrillin-1 cross-link. J. Biol. Chem. 2004, 279, 23748–23758. [Google Scholar] [CrossRef] [PubMed]
- Kagan, H.M.; Cai, P. Isolation of Active Site Peptides of Lysyl Oxidase. Methods Enzymol. 1995, 258, 122–132. [Google Scholar] [CrossRef]
- Keeley, F.W.; Bellingham, C.M.; Woodhouse, K.A. Elastin as a Self-Organizing Biomaterial: Use of Recombinantly Expressed Human Elastin Polypeptides as a Model for Investigations of Structure and Self-Assembly of Elastin. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 185–189. [Google Scholar] [CrossRef]
- Daamen, W.F.; Hafmans, T.; Veerkamp, J.H.; Van Kuppevelt, T.H. Comparison of Five Procedures for the Purification of Insoluble Elastin. Biomaterials 2001, 22, 1997–2005. [Google Scholar] [CrossRef]
- Mecham, R.P. Methods in Elastic Tissue Biology: Elastin Isolation and Purification. Methods 2008, 45, 32–41. [Google Scholar] [CrossRef]
- Sato, F.; Wachi, H.; Starcher, B.C.; Murata, H.; Amano, S.; Tajima, S.; Seyama, Y. The Characteristics of Elastic Fiber Assembled with Recombinant Tropoelastin Isoform. Clin. Biochem. 2006, 39, 746–753. [Google Scholar] [CrossRef]
- Wise, S.G.; Weiss, A.S. Tropoelastin. Int. J. Biochem. Cell Biol. 2009, 41, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Indik, Z.; Abrams, W.R.; Kucich, U.; Gibson, C.W.; Mecham, R.P.; Rosenbloom, J. Production of Recombinant Human Tropoelastin: Characterization and Demonstration of Immunologic and Chemotactic Activity. Arch. Biochem. Biophys. 1990, 280, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Despanie, J.; Dhandhukia, J.P.; Hamm-Alvarez, S.F.; MacKay, J.A. Elastin-like Polypeptides: Therapeutic Applications for an Emerging Class of Nanomedicines. J. Control. Release 2016, 240, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Haller, C.A.; Sallach, R.E.; Chaikof, E.L. Cell Behavior on a CCN1 Functionalized Elastin-Mimetic Protein Polymer. Biomaterials 2012, 33, 2431–2438. [Google Scholar] [CrossRef]
- Jenkins, T.L.; Little, D. Synthetic Scaffolds for Musculoskeletal Tissue Engineering: Cellular Responses to Fiber Parameters. NPJ Regen. Med. 2019, 4, 15. [Google Scholar] [CrossRef]
- Rabionet, M.; Yeste, M.; Puig, T.; Ciurana, J. Electrospinning PCL Scaffolds Manufacture for Three-Dimensional Breast Cancer Cell Culture. Polymers 2017, 9, 328. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Z.; Shen, S.; Liu, G.; Xu, R.X. Combinatory Electrospray and Electrospinning to Produce Multi-Layered Membrane with Enhanced Mechanical Property. Environ. Eng. Res. 2022, 28, 220023. [Google Scholar] [CrossRef]
- Wang, C.; Lu, W.W.; Wang, M. Bicomponent Fibrous Scaffolds Made through Dual-Source Dual-Power Electrospinning: Dual Delivery of RhBMP-2 and Ca-P Nanoparticles and Enhanced Biological Performances. J. Biomed. Mater. Res. Part A 2017, 105, 2199–2209. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Zhao, Q.; Xie, Y.; Yao, X.; Wang, M.; Cao, F. Nanofibrous Bicomponent Scaffolds for the Dual Delivery of NGF and GDNF: Controlled Release of Growth Factors and Their Biological Effects. J. Mater. Sci. Mater. Med. 2021, 32, 9. [Google Scholar] [CrossRef]
- Yoon, J.; Yang, H.-S.; Lee, B.-S.; Yu, W.-R. Recent Progress in Coaxial Electrospinning: New Parameters, Various Structures, and Wide Applications. Adv. Mater. 2018, 30, 1704765. [Google Scholar] [CrossRef]
- Lee, B.-S.; Yang, H.-S.; Yu, W.-R. Fabrication of Double-Tubular Carbon Nanofibers Using Quadruple Coaxial Electrospinning. Nanotechnology 2014, 25, 465602. [Google Scholar] [CrossRef] [PubMed]
- Reise, M.; Kranz, S.; Guellmar, A.; Wyrwa, R.; Rosenbaum, T.; Weisser, J.; Jurke, A.; Schnabelrauch, M.; Heyder, M.; Watts, D.C.; et al. Coaxial Electrospun Nanofibers as Drug Delivery System for Local Treatment of Periodontitis. Dent. Mater. 2023, 39, 132–139. [Google Scholar] [CrossRef]
- Wu, Y.-K.; Wang, L.; Fan, J.; Shou, W.; Zhou, B.-M.; Liu, Y. Multi-Jet Electrospinning with Auxiliary Electrode: The Influence of Solution Properties. Polymers 2018, 10, 572. [Google Scholar] [CrossRef] [PubMed]
- Varesano, A.; Carletto, R.A.; Mazzuchetti, G. Experimental Investigations on the Multi-Jet Electrospinning Process. J. Mater. Process. Technol. 2009, 209, 5178–5185. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion Electrospinning: Fundamentals, Food Applications and Prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Norouzi, M.-R.; Ghasemi-Mobarakeh, L.; Itel, F.; Schoeller, J.; Fashandi, H.; Borzi, A.; Neels, A.; Fortunato, G.; Rossi, R.M. Emulsion Electrospinning of Sodium Alginate/Poly(ε-Caprolactone) Core/Shell Nanofibers for Biomedical Applications. Nanoscale Adv. 2022, 4, 2929–2941. [Google Scholar] [CrossRef]
- Li, X.; Su, Y.; Liu, S.; Tan, L.; Mo, X.; Ramakrishna, S. Encapsulation of Proteins in Poly(l-Lactide-Co-Caprolactone) Fibers by Emulsion Electrospinning. Colloids Surf. B Biointerfaces 2010, 75, 418–424. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larrañeta, E. Development of a Biodegradable Subcutaneous Implant for Prolonged Drug Delivery Using 3D Printing. Pharmaceutics 2020, 12, 105. [Google Scholar] [CrossRef]
- Giri, B.R.; Song, E.S.; Kwon, J.; Lee, J.-H.; Park, J.-B.; Kim, D.W. Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling. Pharmaceutics 2020, 12, 77. [Google Scholar] [CrossRef]
- Xu, X.; Goyanes, A.; Trenfield, S.J.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Gaisford, S.; Basit, A.W. Stereolithography (SLA) 3D Printing of a Bladder Device for Intravesical Drug Delivery. Mater. Sci. Eng. C 2021, 120, 111773. [Google Scholar] [CrossRef]
- Xu, X.; Robles-Martinez, P.; Madla, C.M.; Joubert, F.; Goyanes, A.; Basit, A.W.; Gaisford, S. Stereolithography (SLA) 3D Printing of an Antihypertensive Polyprintlet: Case Study of an Unexpected Photopolymer-Drug Reaction. Addit. Manuf. 2020, 33, 101071. [Google Scholar] [CrossRef]
- Tan, K.H.; Chua, C.K.; Leong, K.F.; Cheah, C.M.; Cheang, P.; Abu Bakar, M.S.; Cha, S.W. Scaffold Development Using Selective Laser Sintering of Polyetheretherketone-Hydroxyapatite Biocomposite Blends. Biomaterials 2003, 24, 3115–3123. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective Laser Sintering Scaffold with Hierarchical Architecture and Gradient Composition for Osteochondral Repair in Rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef]
- Thakkar, R.; Davis, D.A.J.; Williams, R.O.I.I.I.; Maniruzzaman, M. Selective Laser Sintering of a Photosensitive Drug: Impact of Processing and Formulation Parameters on Degradation, Solid State, and Quality of 3D-Printed Dosage Forms. Mol. Pharm. 2021, 18, 3894–3908. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Yang, Y.; Pan, H.; He, C.; Qi, F.; Xie, D.; Liang, H. Selective Laser Melted Fe-Mn Bone Scaffold: Microstructure, Corrosion Behavior and Cell Response. Mater. Res. Express 2020, 7, 15404. [Google Scholar] [CrossRef]
- Su, L.; Jing, L.; Zeng, X.; Chen, T.; Liu, H.; Kong, Y.; Wang, X.; Yang, X.; Fu, C.; Sun, J.; et al. 3D-Printed Prolamin Scaffolds for Cell-Based Meat Culture. Adv. Mater. 2023, 35, 2207397. [Google Scholar] [CrossRef]
- Uehlin, A.F.; Vines, J.B.; Feldman, D.S.; Dean, D.R.; Thomas, V. Inkjet Printing of Nanohydroxyapatite Gradients on Fibrous Scaffold for Bone–Ligament Enthesis. JOM 2022, 74, 3336–3348. [Google Scholar] [CrossRef]
- Kenis, P.J.A.; Ismagilov, R.F.; Whitesides, G.M. Microfabrication inside Capillaries Using Multiphase Laminar Flow Patterning. Science 1999, 285, 83–85. [Google Scholar] [CrossRef]
- Honaker, L.W.; Vats, S.; Anyfantakis, M.; Lagerwall, J.P.F. Elastic Sheath-Liquid Crystal Core Fibres Achieved by Microfluidic Wet Spinning. J. Mater. Chem. C 2019, 7, 11588–11596. [Google Scholar] [CrossRef]
- Haynl, C.; Hofmann, E.; Pawar, K.; Förster, S.; Scheibel, T. Microfluidics-Produced Collagen Fibers Show Extraordinary Mechanical Properties. Nano Lett. 2016, 16, 5917–5922. [Google Scholar] [CrossRef]
- Daniele, M.A.; Boyd, D.A.; Adams, A.A.; Ligler, F.S. Microfluidic Strategies for Design and Assembly of Microfibers and Nanofibers with Tissue Engineering and Regenerative Medicine Applications. Adv. Healthc. Mater. 2015, 4, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, C.; Hu, G.; Xuan, X. Particle Manipulations in Non-Newtonian Microfluidics: A Review. J. Colloid Interface Sci. 2017, 500, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Burrell, G.L.; Dunlop, N.F.; Separovic, F. Non-Newtonian Viscous Shear Thinning in Ionic Liquids. Soft Matter 2010, 6, 2080–2086. [Google Scholar] [CrossRef]
- Zhou, J.; Papautsky, I. Viscoelastic Microfluidics: Progress and Challanges. Microsyst. Nanoeng. 2020, 6, 113. [Google Scholar] [CrossRef]
- Dahlberg, T.; Stangner, T.; Zhang, H.; Wiklund, K.; Lundberg, P.; Edman, L.; Andersson, M. 3D Printed Water-Soluble Scaffolds for Rapid Production of PDMS Micro-Fluidic Flow Chambers. Sci. Rep. 2018, 8, 3372. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.; Waheed, W.; Alamoodi, N.; Mathew, B.; Alnaimat, F.; Abu-Nada, E.; Abderrahmane, A.; Alazzam, A. A Review of Cyclic Olefin Copolymer Applications in Microfluidics and Microdevices. Macromol. Mater. Eng. 2022, 307, 2200053. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Chen, G. Fabrication, Modification, and Application of Poly(Methyl Methacrylate) Microfluidic Chips. Electrophoresis 2008, 29, 1801–1814. [Google Scholar] [CrossRef]
- Navaei-Nigjeh, M.; Mirzababaei, S.; Ghiass, M.A.; Roshanbinfar, K.; Gholami, M.; Abdollahi, M. Microfluidically Fabricated Fibers Containing Pancreatic Islets and Mesenchymal Stromal Cells Improve Longevity and Sustained Normoglycemia in Diabetic Rats. Biofabrication 2023, 15, 15013. [Google Scholar] [CrossRef]
- Nguyen, T.P.T.; Tran, B.M.; Lee, N.Y. Microfluidic Approach for the Fabrication of Cell-Laden Hollow Fibers for Endothelial Barrier Research. J. Mater. Chem. B 2018, 6, 6057–6066. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Y.; Yang, C.; Shao, C.; Shi, K.; Shang, L.; Ye, F.; Zhao, Y. Microfluidic 3D Printing Responsive Scaffolds with Biomimetic Enrichment Channels for Bone Regeneration. Adv. Funct. Mater. 2021, 31, 2105190. [Google Scholar] [CrossRef]
- Du, X.Y.; Li, Q.; Wu, G.; Chen, S. Multifunctional Micro/Nanoscale Fibers Based on Microfluidic Spinning Technology. Adv. Mater. 2019, 31, e1903733. [Google Scholar] [CrossRef] [PubMed]
- Polat, Y.; Pampal, E.S.; Stojanovska, E.; Simsek, R.; Hassanin, A.; Kilic, A.; Demir, A.; Yilmaz, S. Solution Blowing of Thermoplastic Polyurethane Nanofibers: A Facile Method to Produce Flexible Porous Materials. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Cui, Z.; Zhao, L.; Hussain, N.; Zhao, Y.; Yang, C.; Jiang, X.; Li, L.; Song, J.; Zhang, B.; et al. High-Throughput Production of Kilogram-Scale Nanofibers by Kármán Vortex Solution Blow Spinning. Sci. Adv. 2022, 8, eabn3690. [Google Scholar] [CrossRef]
- Jia, C.; Li, L.; Song, J.; Li, Z.; Wu, H. Mass Production of Ultrafine Fibers by a Versatile Solution Blow Spinning Method. Accounts Mater. Res. 2021, 2, 432–446. [Google Scholar] [CrossRef]
- Mary, S.A.; Ariram, N.; Gopinath, A.; Chinnaiyan, S.K.; Raja, I.S.; Sahu, B.; Giri Dev, V.R.; Han, D.-W.; Madhan, B. Investigation on Centrifugally Spun Fibrous PCL/3-Methyl Mannoside Mats for Wound Healing Application. Polymers 2023, 15, 1293. [Google Scholar] [CrossRef]
- Martín-Alonso, M.D.; Salaris, V.; Leonés, A.; Hevilla, V.; Muñoz-Bonilla, A.; Echeverría, C.; Fernández-García, M.; Peponi, L.; López, D. Centrifugal Force-Spinning to Obtain Multifunctional Fibers of PLA Reinforced with Functionalized Silver Nanoparticles. Polymers 2023, 15, 1240. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y. Centrifugal Spinning: An Alternative Approach to Fabricate Nanofibers at High Speed and Low Cost. Polym. Rev. 2014, 54, 677–701. [Google Scholar] [CrossRef]
- Amariei, N.; Manea, L.R.; Bertea, A.P.; Bertea, A.; Popa, A. The Influence of Polymer Solution on the Properties of Electrospun 3D Nanostructures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012092. [Google Scholar] [CrossRef]
- Bakar, S.S.S.; Fong, K.C.; Eleyas, A.; Nazeri, M.F.M. Effect of Voltage and Flow Rate Electrospinning Parameters on Polyacrylonitrile Electrospun Fibers. IOP Conf. Ser. Mater. Sci. Eng. 2018, 318, 012076. [Google Scholar] [CrossRef]
- Zhong, H.; Huang, J.; Wu, J.; Du, J. Electrospinning Nanofibers to 1D, 2D, and 3D Scaffolds and Their Biomedical Applications. Nano Res. 2022, 15, 787–804. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of Polymeric Nanofibers for Drug Delivery Applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Balaji, A.; Vellayappan, M.V.; John, A.A.; Subramanian, A.P.; Jaganathan, S.K.; Supriyanto, E.; Razak, S.I.A. An Insight on Electrospun-Nanofibers-Inspired Modern Drug Delivery System in the Treatment of Deadly Cancers. RSC Adv. 2015, 5, 57984–58004. [Google Scholar] [CrossRef]
- Schaub, N.J.; Corey, J.M. A Method to Rapidly Analyze the Simultaneous Release of Multiple Pharmaceuticals from Electrospun Fibers. Int. J. Pharm. 2020, 574, 118871. [Google Scholar] [CrossRef] [PubMed]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-Based Systems for Fabrication of Electrospun Nanofibers: Food, Pharmaceutical and Biomedical Applications. RSC Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef]
- Xue, J.; Niu, Y.; Gong, M.; Shi, R.; Chen, D.; Zhang, L.; Lvov, Y. Electrospun Microfiber Membranes Embedded with Drug-Loaded Clay Nanotubes for Sustained Antimicrobial Protection. ACS Nano 2015, 9, 1600–1612. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, P.; Zhang, A.; Qin, Z.; Li, Y.; Xianyu, Y.; Zhang, H. Covalent Organic Framework-Incorporated Nanofibrous Membrane as an Intelligent Platform for Wound Dressing. ACS Appl. Mater. Interfaces 2022, 14, 8680–8692. [Google Scholar] [CrossRef]
- Lategan, M.; Kumar, P.; Choonara, Y.E. Functionalizing Nanofibrous Platforms for Neural Tissue Engineering Applications. Drug Discov. Today 2022, 27, 1381–1403. [Google Scholar] [CrossRef]
- Kono, H.; Uno, T.; Tsujisaki, H.; Anai, H.; Kishimoto, R.; Matsushima, T.; Tajima, K. Nanofibrillated Bacterial Cellulose Surface Modified with Methyltrimethoxysilane for Fiber-Reinforced Composites. ACS Appl. Nano Mater. 2020, 3, 8232–8241. [Google Scholar] [CrossRef]
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A Review on Electrospun Nanofibers Based Advanced Applications: From Health Care to Energy Devices. Polymers 2021, 13, 3746. [Google Scholar] [CrossRef]
- Zhong, H.; Li, Z.; Zhao, T.; Chen, Y. Surface Modification of Nanofibers by Physical Adsorption of Fiber-Homologous Amphiphilic Copolymers and Nanofiber-Reinforced Hydrogels with Excellent Tissue Adhesion. ACS Biomater. Sci. Eng. 2021, 7, 4828–4837. [Google Scholar] [CrossRef]
- Kumari, P.; Meena, A. Application of Enzyme-Mediated Cellulose Nanofibers from Lemongrass Waste for the Controlled Release of Anticancer Drugs. Environ. Sci. Pollut. Res. 2021, 28, 46343–46355. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Greiner, A. On the way to clean and safe electrospinning-green electrospinning: Emulsion and suspension electrospinning. Polym. Adv. Technol. 2011, 22, 372–378. [Google Scholar] [CrossRef]
- Li, J.; Xu, W.; Li, D.; Liu, T.; Zhang, Y.S.; Ding, J.; Chen, X. Locally Deployable Nanofiber Patch for Sequential Drug Delivery in Treatment of Primary and Advanced Orthotopic Hepatomas. ACS Nano 2018, 12, 6685–6699. [Google Scholar] [CrossRef]
- Xia, G.; Zhang, H.; Cheng, R.; Wang, H.; Song, Z.; Deng, L.; Huang, X.; Santos, H.A.; Cui, W. Localized Controlled Delivery of Gemcitabine via Microsol Electrospun Fibers to Prevent Pancreatic Cancer Recurrence. Adv. Healthc. Mater. 2018, 7, e1800593. [Google Scholar] [CrossRef]
- Liao, I.C.; Chew, S.Y.; Leong, K.W. Aligned Core-Shell Nanofibers Delivering Bioactive Proteins. Nanomedicine 2006, 1, 465–471. [Google Scholar] [CrossRef]
- Lan, X.; Wang, H.; Bai, J.; Miao, X.; Lin, Q.; Zheng, J.; Ding, S.; Li, X.; Tang, Y. Multidrug-Loaded Electrospun Micro/Nanofibrous Membranes: Fabrication Strategies, Release Behaviors and Applications in Regenerative Medicine. J. Control. Release 2021, 330, 1264–1287. [Google Scholar] [CrossRef]
- Darbasizadeh, B.; Mortazavi, S.A.; Kobarfard, F.; Jaafari, M.R.; Hashemi, A.; Farhadnejad, H.; Feyzi-barnaji, B. Electrospun Doxorubicin-Loaded PEO/PCL Core/Sheath Nanofibers for Chemopreventive Action against Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2021, 64, 102576. [Google Scholar] [CrossRef]
- Rui, J.; Li, J.; Li, Y.; Liu, H.; Zhang, Y.; Huang, J.; Ma, P.; Chen, Z. Preparation of C–SiC/ZrSixOyCz Core-Shell Fibers by Coaxial Electrospinning and the High-Temperature Performance. Ceram. Int. 2022, 48, 2625–2631. [Google Scholar] [CrossRef]
- Boda, S.K.; Chen, S.; Chu, K.; Kim, H.J.; Xie, J. Electrospraying Electrospun Nanofiber Segments into Injectable Microspheres for Potential Cell Delivery. ACS Appl. Mater. Interfaces 2018, 10, 25069–25079. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Qiu, J.; Rutledge, S.; Tanes, M.L.; Xia, Y. Promoting Cell Migration and Neurite Extension along Uniaxially Aligned Nanofibers with Biomacromolecular Particles in a Density Gradient. Adv. Funct. Mater. 2020, 30, 2002031. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Kwon, Y.W.; Lee, T.W.; Park, G.T.; Kim, J.H. Recent Advances in Stem Cell Therapeutics and Tissue Engineering Strategies. Biomater. Res. 2018, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, P.R.; Dwyer, K.D.; Coulombe, K.L.K. Current Applications of Polycaprolactone as a Scaffold Material for Heart Regeneration. ACS Appl. Bio Mater. 2022, 5, 2461–2480. [Google Scholar] [CrossRef] [PubMed]
- Sunil Richard, A.; Verma, R.S. Antioxidant α-Mangostin Coated Woven Polycaprolactone Nanofibrous Yarn Scaffold for Cardiac Tissue Repair. ACS Appl. Nano Mater. 2022, 5, 5075–5086. [Google Scholar] [CrossRef]
- Wu, S.; Dong, T.; Li, Y.; Sun, M.; Qi, Y.; Liu, J.; Kuss, M.A.; Chen, S.; Duan, B. State-of-the-Art Review of Advanced Electrospun Nanofiber Yarn-Based Textiles for Biomedical Applications. Appl. Mater. Today 2022, 27, 101473. [Google Scholar] [CrossRef]
- Farías, J.G.; Molina, V.M.; Carrasco, R.A.; Zepeda, A.B.; Figueroa, E.; Letelier, P.; Castillo, R.L. Antioxidant Therapeutic Strategies for Cardiovascular Conditions Associated with Oxidative Stress. Nutrients 2017, 9, 966. [Google Scholar] [CrossRef]
- Herrera-Aco, D.R.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Sciutto-Conde, E.; Rosas-Salgado, G.; Fragoso-González, G. Alpha-Mangostin: Anti-Inflammatory and Antioxidant Effects on Established Collagen-Induced Arthritis in DBA/1J Mice. Food Chem. Toxicol. 2019, 124, 300–315. [Google Scholar] [CrossRef]
- Tao, G.; Kahr, P.C.; Morikawa, Y.; Zhang, M.; Rahmani, M.; Heallen, T.R.; Li, L.; Sun, Z.; Olson, E.N.; Amendt, B.A.; et al. Pitx2 Promotes Heart Repair by Activating the Antioxidant Response after Cardiac Injury. Nature 2016, 534, 119–123. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Dabiri, A.E.; Kassab, G.S. Thrombogenic and Inflammatory Reactions to Biomaterials in Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 123. [Google Scholar] [CrossRef]
- Boccafoschi, F.; Mosca, C.; Cannas, M. Cardiovascular Biomaterials: When the Inflammatory Response Helps to Efficiently Restore Tissue Functionality? J. Tissue Eng. Regen. Med. 2014, 8, 253–267. [Google Scholar] [CrossRef]
- Guo, M.; Wang, X.; Liu, Y.; Yu, H.; Dong, J.; Cui, Z.; Bai, Z.; Li, K.; Li, Q. Hierarchical Shish–Kebab Structures Functionalizing Nanofibers for Controlled Drug Release and Improved Antithrombogenicity. Biomacromolecules 2022, 23, 1337–1349. [Google Scholar] [CrossRef]
- Hirsh, J.; Fuster, V. Guide to Anticoagulant Therapy. Part 1: Heparin. Circulation 1994, 89, 1449–1468. [Google Scholar] [CrossRef] [PubMed]
- Biran, R.; Pond, D. Heparin Coatings for Improving Blood Compatibility of Medical Devices. Adv. Drug Deliv. Rev. 2017, 112, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Onishi, A.; St Ange, K.; Dordick, J.S.; Linhardt, R.J. Heparin and Anticoagulation. Front. Biosci.-Landmark 2016, 21, 1372–1392. [Google Scholar] [CrossRef]
- Chen, J.; Verni, C.C.; Jouppila, A.; Lassila, R.; Diamond, S.L. Dual Antiplatelet and Anticoagulant (APAC) Heparin Proteoglycan Mimetic with Shear-Dependent Effects on Platelet-Collagen Binding and Thrombin Generation. Thromb. Res. 2018, 169, 143–151. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Wang, X.-C.; Peng, X.-F.; Turng, L.-S. Shish-Kebab-Structured Poly(ε-Caprolactone) Nanofibers Hierarchically Decorated with Chitosan–Poly(ε-Caprolactone) Copolymers for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 6955–6965. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Li, X.; Jiang, Y.C.; Han, S.; Ma, L.; Guo, H.; Wang, Z.; Li, Q. Endothelial Cell Migration on Poly(ϵ-Caprolactone) Nanofibers Coated with a Nanohybrid Shish-Kebab Structure Mimicking Collagen Fibrils. Biomacromolecules 2020, 21, 1202–1213. [Google Scholar] [CrossRef]
- Sun, X.; Altalhi, W.; Nunes, S.S. Vascularization Strategies of Engineered Tissues and Their Application in Cardiac Regeneration. Adv. Drug Deliv. Rev. 2016, 96, 183–194. [Google Scholar] [CrossRef]
- Singh, S.; Prakash, S.; Gupta, S.K. Angiogenesis: A Critical Determinant for Cardiac Regeneration. Mol. Ther.-Nucleic Acids 2022, 29, 88–89. [Google Scholar] [CrossRef]
- Meng, J.; Xiao, B.; Wu, F.; Sun, L.; Li, B.; Guo, W.; Hu, X.; Xu, X.; Wen, T.; Liu, J.; et al. Co-Axial Fibrous Scaffolds Integrating with Carbon Fiber Promote Cardiac Tissue Regeneration Post Myocardial Infarction. Mater. Today Bio 2022, 16, 100415. [Google Scholar] [CrossRef]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef]
- Yang, G.; Mahadik, B.; Choi, J.Y.; Fisher, J.P. Vascularization in Tissue Engineering: Fundamentals and State-of-Art. Prog. Biomed. Eng. 2020, 2, 012002. [Google Scholar] [CrossRef]
- Varadarajan, S.G.; Hunyara, J.L.; Hamilton, N.R.; Kolodkin, A.L.; Huberman, A.D. Central Nervous System Regeneration. Cell 2022, 185, 77–94. [Google Scholar] [CrossRef]
- Thomas, C.K.; Zaidner, E.Y.; Calancie, B.; Broton, J.G.; Bigland-Ritchie, B.R. Muscle Weakness, Paralysis, and Atrophy after Human Cervical Spinal Cord Injury. Exp. Neurol. 1997, 148, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Peressotti, S.; Koehl, G.E.; Goding, J.A.; Green, R.A. Self-Assembling Hydrogel Structures for Neural Tissue Repair. ACS Biomater. Sci. Eng. 2021, 7, 4136–4163. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Sun, C. The Roles of MicroRNAs in Neural Regenerative Medicine. Exp. Neurol. 2020, 332, 113394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Milbreta, U.; Chin, J.S.; Pinese, C.; Lin, J.; Shirahama, H.; Jiang, W.; Liu, H.; Mi, R.; Hoke, A.; et al. Biomimicking Fiber Scaffold as an Effective In Vitro and In Vivo MicroRNA Screening Platform for Directing Tissue Regeneration. Adv. Sci. 2019, 6, 1800808. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Diao, H.J.; Chew, S.Y. MicroRNAs and Their Potential Therapeutic Applications in Neural Tissue Engineering. Adv. Drug Deliv. Rev. 2015, 88, 53–66. [Google Scholar] [CrossRef]
- Dong, X.; Wu, P.; Yan, L.; Liu, K.; Wei, W.; Cheng, Q.; Liang, X.; Chen, Y.; Dai, H. Oriented Nanofibrous P(MMD-Co-LA)/Deferoxamine Nerve Scaffold Facilitates Peripheral Nerve Regeneration by Regulating Macrophage Phenotype and Revascularization. Biomaterials 2022, 280, 121288. [Google Scholar] [CrossRef]
- Holden, P.; Nair, L.S. Deferoxamine: An Angiogenic and Antioxidant Molecule for Tissue Regeneration. Tissue Eng.-Part B Rev. 2019, 25, 461–470. [Google Scholar] [CrossRef]
- Ikeda, Y.; Tajima, S.; Yoshida, S.; Yamano, N.; Kihira, Y.; Ishizawa, K.; Aihara, K.I.; Tomita, S.; Tsuchiya, K.; Tamaki, T. Deferoxamine Promotes Angiogenesis via the Activation of Vascular Endothelial Cell Function. Atherosclerosis 2011, 215, 339–347. [Google Scholar] [CrossRef]
- Jiang, H.; Qian, Y.; Fan, C.; Ouyang, Y. Polymeric Guide Conduits for Peripheral Nerve Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 582646. [Google Scholar] [CrossRef] [PubMed]
- White, D.T.; Sengupta, S.; Saxena, M.T.; Xu, Q.; Hanes, J.; Ding, D.; Ji, H.; Mumm, J.S. Immunomodulation-Accelerated Neuronal Regeneration Following Selective Rod Photoreceptor Cell Ablation in the Zebrafish Retina. Proc. Natl. Acad. Sci. USA 2017, 114, E3719–E3728. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1-M2 Polarization Balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Zou, X.B.; Chai, Y.F.; Yao, Y.M. Macrophage Polarization in Inflammatory Diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef]
- Xi, K.; Gu, Y.; Tang, J.; Chen, H.; Xu, Y.; Wu, L.; Cai, F.; Deng, L.; Yang, H.; Shi, Q.; et al. Microenvironment-Responsive Immunoregulatory Electrospun Fibers for Promoting Nerve Function Recovery. Nat. Commun. 2020, 11, 4504. [Google Scholar] [CrossRef]
- Ansari, M. Bone Tissue Regeneration: Biology, Strategies and Interface Studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Geng, M.; Zhang, Q.; Gu, J.; Yang, J.; Du, H.; Jia, Y.; Zhou, X.; He, C. Construction of a Nanofiber Network within 3D Printed Scaffolds for Vascularized Bone Regeneration. Biomater. Sci. 2021, 9, 2631–2646. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Coaxial Electrospinning Formation of Complex Polymer Fibers and Their Applications. Chempluschem 2019, 84, 1453–1497. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Duarte, A.R.C.; Faria, S.; Marques, A.P.; Reis, R.L.; Neves, N.M. Osteogenic Induction of HBMSCs by Electrospun Scaffolds with Dexamethasone Release Functionality. Biomaterials 2010, 31, 5875–5885. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, M.; Yamada, T.; Taniyama, T.; Masaoka, T.; Xuetao, W.; Yoshii, T.; Horie, M.; Yasuda, H.; Uemura, T.; Okawa, A.; et al. Dexamethasone Enhances Osteogenic Differentiation of Bone Marrow-and Muscle-Derived Stromal Cells and Augments Ectopic Bone Formation Induced by Bone Morphogenetic Protein-2. PLoS ONE 2015, 10, e0116462. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Fang, J.; Zhong, C.; Wang, M.; Ren, F. Spatiotemporal Delivery of PBMP2 and PVEGF by a Core–Sheath Structured Fiber-Hydrogel Gene-Activated Matrix Loaded with Peptide-Modified Nanoparticles for Critical-Sized Bone Defect Repair. Adv. Healthc. Mater. 2022, 11, 2201096. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.; Li, C.; Yu, Y. Role of Bone Morphogenetic Protein-2 in Osteogenic Differentiation of Mesenchymal Stem Cells. Mol. Med. Rep. 2015, 12, 4230–4237. [Google Scholar] [CrossRef]
- Lu, L.Y.; Loi, F.; Nathan, K.; Lin, T.H.; Pajarinen, J.; Gibon, E.; Nabeshima, A.; Cordova, L.; Jämsen, E.; Yao, Z.; et al. Pro-Inflammatory M1 Macrophages Promote Osteogenesis by Mesenchymal Stem Cells via the COX-2-Prostaglandin E2 Pathway. J. Orthop. Res. 2017, 35, 2378–2385. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Risser, G.E.; Spiller, K.L. Sequential Drug Delivery to Modulate Macrophage Behavior and Enhance Implant Integration. Adv. Drug Deliv. Rev. 2019, 149–150, 85–94. [Google Scholar] [CrossRef]
- He, M.; Wang, Q.; Feng, Y.; Gao, X.; He, C.; Li, J.; Zhao, W.; Tian, W.; Zhao, C. Spatiotemporal Management of the Osteoimmunomodulation of Fibrous Scaffolds by Loading a Novel Amphiphilic Nanomedicine. ACS Appl. Mater. Interfaces 2022, 14, 13991–14003. [Google Scholar] [CrossRef]
- Nalamachu, S.; Wortmann, R. Role of Indomethacin in Acute Pain and Inflammation Management: A Review of the Literature. Postgrad. Med. 2014, 126, 92–97. [Google Scholar] [CrossRef]
- Yeo, J.; Lee, J.; Yoon, S.; Kim, W.J. Tannic Acid-Based Nanogel as an Efficient Anti-Inflammatory Agent. Biomater. Sci. 2020, 8, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and Its Impact on the Immune System. Curr. Diabetes Rev. 2019, 16, 442–449. [Google Scholar] [CrossRef]
- Kandhasamy, S.; Liang, B.; Yang, D.P.; Zeng, Y. Antibacterial Vitamin K3 Carnosine Peptide-Laden Silk Fibroin Electrospun Fibers for Improvement of Skin Wound Healing in Diabetic Rats. ACS Appl. Bio Mater. 2021, 4, 4769–4788. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Feng, Z.; Han, B.; Yu, D.G.; Wang, K. Advances in the Preparation of Nanofiber Dressings by Electrospinning for Promoting Diabetic Wound Healing. Biomolecules 2022, 12, 1727. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Gradys, A.; Sajkiewicz, P. Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering. Polymers 2020, 12, 2636. [Google Scholar] [CrossRef]
- Nosrati, H.; Aramideh Khouy, R.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite Scaffolds for Accelerating Chronic Wound Healing by Enhancing Angiogenesis. J. Nanobiotechnology 2021, 19, 1–21. [Google Scholar] [CrossRef]
- Graham, M.L.; Janecek, J.L.; Kittredge, J.A.; Hering, B.J.; Schuurman, H.J. The Streptozotocin-Induced Diabetic Nude Mouse Model: Differences between Animals from Different Sources. Comp. Med. 2011, 61, 356–360. [Google Scholar] [PubMed]
- Guo, S.; DiPietro, L.A. Critical Review in Oral Biology & Medicine: Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Eming, S.A.; Werner, S.; Bugnon, P.; Wickenhauser, C.; Siewe, L.; Utermöhlen, O.; Davidson, J.M.; Krieg, T.; Roers, A. Accelerated Wound Closure in Mice Deficient for Interleukin-10. Am. J. Pathol. 2007, 170, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Kieran, I.; Knock, A.; Bush, J.; So, K.; Metcalfe, A.; Hobson, R.; Mason, T.; O’Kane, S.; Ferguson, M. Interleukin-10 Reduces Scar Formation in Both Animal and Human Cutaneous Wounds: Results of Two Preclinical and Phase II Randomized Control Studies. Wound Repair Regen. 2013, 21, 428–436. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Balaji, S.; Le, L.D.; Crombleholme, T.M.; Keswani, S.G. Regenerative Wound Healing: The Role of Interleukin-10. Adv. Wound Care 2014, 3, 315–323. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Zhang, H.; Sun, X.; Liu, D.; Zhang, J.; Zhang, Y.; Cheng, L.; Santos, H.A.; Cui, W. Programmable Immune Activating Electrospun Fibers for Skin Regeneration. Bioact. Mater. 2021, 6, 3218–3230. [Google Scholar] [CrossRef]
- Cojocaru, E.; Ghitman, J.; Stan, R. Electrospun-Fibrous-Architecture-Mediated Non-Viral Gene Therapy Drug Delivery in Regenerative Medicine. Polymers 2022, 14, 2647. [Google Scholar] [CrossRef]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The Potential of Nitric Oxide Releasing Therapies as Antimicrobial Agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Schoenfisch, M.H. Nitric Oxide Release: Part II. Therapeutic Applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Aveyard, J.; Doherty, K.G.; Deller, R.C.; Williams, R.L.; Kolegraff, K.N.; Kaye, S.B.; D’Sa, R.A. Antimicrobial Nitric Oxide-Releasing Electrospun Dressings for Wound Healing Applications. ACS Mater. Au 2022, 2, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D.A. Mechanism and Kinetics of the Crosslinking Reaction between Biopolymers Containing Primary Amine Groups and Genipin. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Lien, S.M.; Li, W.T.; Huang, T.J. Genipin-Crosslinked Gelatin Scaffolds for Articular Cartilage Tissue Engineering with a Novel Crosslinking Method. Mater. Sci. Eng. C 2008, 28, 36–43. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, J.; Lee, Y.M.; Im, S.; Park, H.; Kim, W.J. Nitric Oxide-Releasing Polymer Incorporated Ointment for Cutaneous Wound Healing. J. Control. Release 2015, 220, 624–630. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Singha, K.; Han, D.K.; Park, H.; Kim, W.J. Nitric Oxide Integrated Polyethylenimine-Based Tri-Block Copolymer for Efficient Antibacterial Activity. Biomaterials 2013, 34, 8766–8775. [Google Scholar] [CrossRef]
- Maragos, C.M.; Morley, D.; Wink, D.A.; Dunams, T.M.; Saavedra, J.E.; Hoffman, A.; Bove, A.A.; Isaac, L.; Hrabie, J.A.; Keefer, L.K. Complexes of.NO with Nucleophiles as Agents for the Controlled Biological Release of Nitric Oxide. Vasorelaxant Effects. J. Med. Chem. 1991, 34, 3242–3247. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Yahya, E.B.; Alqadhi, A.M. Recent Trends in Cancer Therapy: A Review on the Current State of Gene Delivery. Life Sci. 2021, 269, 119087. [Google Scholar] [CrossRef]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.F.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional Surgery for Cancer Cure: Therapy at a Cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Massagué, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Zaszczyńska, A.; Niemczyk-Soczynska, B.; Sajkiewicz, P. A Comprehensive Review of Electrospun Fibers, 3D-Printed Scaffolds, and Hydrogels for Cancer Therapies. Polymers 2022, 14, 5278. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Z.; Wang, H.; Luo, X.; Xu, Y.; Chan, H.F.; Lv, S.; Tao, Y.; Li, M. Implantable Sandwich-like Scaffold/Fiber Composite Spatiotemporally Releasing Combretastatin A4 and Doxorubicin for Efficient Inhibition of Postoperative Tumor Recurrence. ACS Appl. Mater. Interfaces 2022, 14, 27525–27537. [Google Scholar] [CrossRef]
- Zimmerli, W.; Sendi, P. Pathogenesis of Implant-Associated Infection: The Role of the Host. Semin. Immunopathol. 2011, 33, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential Role of Intratumor Bacteria in Mediating Tumor Resistance to the Chemotherapeutic Drug Gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, Y.; Liang, B.; Suo, D.; Lyu, S.; Wang, Y.; Zhao, X. An Anti-Bacterial and Anti-Cancer Fibrous Membrane with Multiple Therapeutic Effects for Prevention of Pancreatic Cancer Recurrence. Biomater. Adv. 2022, 137, 212831. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Borkowski, M.; Barzowska, A.; Kobielusz, M.; Dąbrowski, J.M. Synthesis and Characterization of Size- and Charge-Tunable Silver Nanoparticles for Selective Anticancer and Antibacterial Treatment. ACS Appl. Mater. Interfaces 2022, 14, 14981–14996. [Google Scholar] [CrossRef]
- Shang, L.; Liu, Z.; Ma, B.; Shao, J.; Wang, B.; Ma, C.; Ge, S. Dimethyloxallyl Glycine/Nanosilicates-Loaded Osteogenic/Angiogenic Difunctional Fibrous Structure for Functional Periodontal Tissue Regeneration. Bioact. Mater. 2021, 6, 1175–1188. [Google Scholar] [CrossRef]
- Cho, Y.D.; Kim, K.H.; Lee, Y.M.; Ku, Y.; Seol, Y.J. Periodontal Wound Healing and Tissue Regeneration: A Narrative Review. Pharmaceuticals 2021, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.; Caton, J.; Benoit, D.S.W. Periodontal Wound Healing and Regeneration: Insights for Engineering New Therapeutic Approaches. Front. Dent. Med. 2022, 3, 5810. [Google Scholar] [CrossRef]
- Sethiya, K.R.; Dhadse, P.V. Healing after Periodontal Surgery—A Review. J. Evol. Med. Dent. Sci. 2020, 9, 3753–3759. [Google Scholar] [CrossRef]
- Vasiliadis, A.V.; Katakalos, K. The Role of Scaffolds in Tendon Tissue Engineering. J. Funct. Biomater. 2020, 11, 78. [Google Scholar] [CrossRef]
- Gaspar, D.; Spanoudes, K.; Holladay, C.; Pandit, A.; Zeugolis, D. Progress in Cell-Based Therapies for Tendon Repair. Adv. Drug Deliv. Rev. 2015, 84, 240–256. [Google Scholar] [CrossRef]
- Chen, J.; Sheng, D.; Ying, T.; Zhao, H.; Zhang, J.; Li, Y.; Xu, H.; Chen, S. MOFs-Based Nitric Oxide Therapy for Tendon Regeneration. Nano-Micro Lett. 2021, 13, 23. [Google Scholar] [CrossRef]
- Mao, Z.; Fan, B.; Wang, X.; Huang, X.; Guan, J.; Sun, Z.; Xu, B.; Yang, M.; Chen, Z.; Jiang, D.; et al. A Systematic Review of Tissue Engineering Scaffold in Tendon Bone Healing in Vivo. Front. Bioeng. Biotechnol. 2021, 9, 621483. [Google Scholar] [CrossRef]
- Liu, S.; Yao, L.; Wang, Y.; Li, Y.; Jia, Y.; Yang, Y.; Li, N.; Hu, Y.; Kong, D.; Dong, X.; et al. Immunomodulatory Hybrid Micro-Nanofiber Scaffolds Enhance Vascular Regeneration. Bioact. Mater. 2023, 21, 464–482. [Google Scholar] [CrossRef]
- Campbell, J.D.; Lakshmanan, R.; Selvaraju, V.; Accorsi, D.; McFadden, D.W.; Maulik, N.; Thirunavukkarasu, M. Engineered Resveratrol-Loaded Fibrous Scaffolds Promotes Functional Cardiac Repair and Regeneration through Thioredoxin-1 Mediated VEGF Pathway. Int. J. Pharm. 2021, 597, 120236. [Google Scholar] [CrossRef]
- Xie, J.; Li, J.; Ma, J.; Li, M.; Wang, X.; Fu, X.; Ma, Y.; Yang, H.; Li, B. Saijilafu Magnesium Oxide/Poly(l-Lactide-Co-ε-Caprolactone) Scaffolds Loaded with Neural Morphogens Promote Spinal Cord Repair through Targeting the Calcium Influx and Neuronal Differentiation of Neural Stem Cells. Adv. Healthc. Mater. 2022, 11, e2200386. [Google Scholar] [CrossRef]
- Jin, B.; Yu, Y.; Lou, C.; Zhang, X.; Gong, B.; Chen, J.; Chen, X.; Zhou, Z.; Zhang, L.; Xiao, J.; et al. Combining a Density Gradient of Biomacromolecular Nanoparticles with Biological Effectors in an Electrospun Fiber-Based Nerve Guidance Conduit to Promote Peripheral Nerve Repair. Adv. Sci. 2022, 10, 2203296. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xi, K.; Tang, J.; Wang, J.; Tang, Y.; Wu, L.; Xu, Y.; Xu, Z.; Chen, L.; Cui, W.; et al. Engineered Living Oriented Electrospun Fibers Regulate Stem Cell Para-Secretion and Differentiation to Promote Spinal Cord Repair. Adv. Healthc. Mater. 2022, 12, 2202785. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, D.P.; Kim, M.H.; Park, H.; Park, W.H.; Kim, B.S.; Kim, C.S. Coaxially Fabricated Polylactic Acid Electrospun Nanofibrous Scaffold for Sequential Release of Tauroursodeoxycholic Acid and Bone Morphogenic Protein2 to Stimulate Angiogenesis and Bone Regeneration. Chem. Eng. J. 2020, 389, 123470. [Google Scholar] [CrossRef]
- Wang, C.; Lu, W.W.; Wang, M. Multifunctional Fibrous Scaffolds for Bone Regeneration with Enhanced Vascularization. J. Mater. Chem. B 2020, 8, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, L.; Tang, Y.; Xi, K.; Tang, J.; Xu, Y.; Xu, J.; Lu, J.; Guo, K.; Gu, Y.; et al. Spatiotemporal Regulation of the Bone Immune Microenvironment via Dam-Like Biphasic Bionic Periosteum for Bone Regeneration. Adv. Healthc. Mater. 2023, 12, 2201661. [Google Scholar] [CrossRef]
- Alyamani, A.A.; Al-Musawi, M.H.; Albukhaty, S.; Sulaiman, G.M.; Ibrahim, K.M.; Ahmed, E.M.; Jabir, M.S.; Al-Karagoly, H.; Aljahmany, A.A.; Mohammed, M.K.A. Electrospun Polycaprolactone/Chitosan Nanofibers Containing Cordia Myxa Fruit Extract as Potential Biocompatible Antibacterial Wound Dressings. Molecules 2023, 28, 2501. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M.; Wang, X.; Zhang, M.; Wang, X.; Li, Y.; Cui, Z.; Chen, X.; Han, Y.; Zhao, W. Electrospun Multifunctional Nanofibrous Mats Loaded with Bioactive Anemoside B4 for Accelerated Wound Healing in Diabetic Mice. Drug Deliv. 2022, 29, 174–185. [Google Scholar] [CrossRef]
- Alizadehgiashi, M.; Nemr, C.R.; Chekini, M.; Pinto Ramos, D.; Mittal, N.; Ahmed, S.U.; Khuu, N.; Kelley, S.O.; Kumacheva, E. Multifunctional 3D-Printed Wound Dressings. ACS Nano 2021, 15, 12375–12387. [Google Scholar] [CrossRef]
- Gong, W.; Huang, H.B.; Wang, X.C.; He, W.Y.; Hu, J.N. Coassembly of Fiber Hydrogel with Antibacterial Activity for Wound Healing. ACS Biomater. Sci. Eng. 2023, 9, 375–387. [Google Scholar] [CrossRef]
- Wu, X.; Chen, J.; Zhou, A.; Zhao, Y.; Tian, Z.; Zhang, Y.; Chen, K.; Ning, X.; Xu, Y. Light-Activated Chemically Reactive Fibrous Patch Revolutionizes Wound Repair Through the Prevention of Postoperative Adhesion. Nano Lett. 2022, 23, 1435–1444. [Google Scholar] [CrossRef]
- Yu, M.; Tang, P.; Tang, Y.; Wei, C.; Wang, Z.; Zhang, H. Breathable, Moisturizing, Anti-Oxidation SSD-PG-PVA/KGM Fibrous Membranes for Accelerating Diabetic Wound Tissue Regeneration. ACS Appl. Bio Mater. 2022, 5, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Ajmal, G.; Upadhyay, S.N.; Mishra, P.K. Nano-Fibrous Scaffold with Curcumin for Anti-Scar Wound Healing. Int. J. Pharm. 2020, 589, 119858. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, A.; Chauhan, N.S.; Yadav, G.; Goswami, M.; Packirisamy, G. Design and Fabrication of a Dual Protein-Based Trilayered Nanofibrous Scaffold for Efficient Wound Healing. ACS Appl. Bio Mater. 2022, 5, 2726–2740. [Google Scholar] [CrossRef]
- Fu, Y.; Cen, D.; Zhang, T.; Jiang, S.; Wang, Y.; Cai, X.; Li, X.; Han, G. Implantable Fibrous Scaffold with Hierarchical Microstructure for the ‘on-Site’ Synergistic Cancer Therapy. Chem. Eng. J. 2020, 402, 126204. [Google Scholar] [CrossRef]
- Wei, X.; Liu, C.; Wang, Z.; Luo, Y. 3D Printed Core-Shell Hydrogel Fiber Scaffolds with NIR-Triggered Drug Release for Localized Therapy of Breast Cancer. Int. J. Pharm. 2020, 580, 19219. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, D.; Tang, Y.; Guo, Z.; Lin, K.; Yu, Y.; Li, J.; Cai, Q. Fibrous Dressing Containing Bioactive Glass with Combined Chemotherapy and Wound Healing Promotion for Post-Surgical Treatment of Melanoma. Biomater. Adv. 2023, 149, 213387. [Google Scholar] [CrossRef]
- Echeverria Molina, M.I.; Malollari, K.G.; Komvopoulos, K. Design Challenges in Polymeric Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 617141. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Zhang, H.; Zhuang, Y.; Shen, H.; Chen, Y.; Zhao, Y.; Chen, B.; Xiao, Z.; Dai, J. Aligned Scaffolds with Biomolecular Gradients for Regenerative Medicine. Polymers 2019, 11, 341. [Google Scholar] [CrossRef]
- Owida, H.A.; Al-Nabulsi, J.I.; Alnaimat, F.; Al-Ayyad, M.; Turab, N.M.; Al Sharah, A.; Shakur, M. Recent Applications of Electrospun Nanofibrous Scaffold in Tissue Engineering. Appl. Bionics Biomech. 2022, 2022, 1953861. [Google Scholar] [CrossRef]
- Webber, M.J.; Khan, O.F.; Sydlik, S.A.; Tang, B.C.; Langer, R. A Perspective on the Clinical Translation of Scaffolds for Tissue Engineering. Ann. Biomed. Eng. 2015, 43, 641–656. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Zanjanizadeh Ezazi, N.; Liu, D.; Santos, H.A. Electrospun Fibrous Architectures for Drug Delivery, Tissue Engineering and Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1802852. [Google Scholar] [CrossRef]
- Nayl, A.; Abd-Elhamid, A.; Awwad, N.; Abdelgawad, M.; Wu, J.; Mo, X.; Gomha, S.; Aly, A.; Brase, S. Recent Progress and Potential Biomedical Applications of Electrospun Nanofibers in Regeneration of Tissues and Organs. Polymers 2022, 14, 1508. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Ishikawa, F.; Greiner, D.L. Humanized Mice in Translational Biomedical Research. Nat. Rev. Immunol. 2007, 7, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.M.; He, J.; Xu, Y.; Christman, K.L. Humanized Mouse Model for Evaluating Biocompatibility and Human Immune Cell Interactions to Biomaterials. Drug Discov. Today Dis. Model. 2017, 24, 23–29. [Google Scholar] [CrossRef]



















| Spinning Method | Key Features | Applications |
|---|---|---|
| Bicomponent spinning [79,80] | Two polymers are spun together to create a core–sheath or side-by-side structure | Medical textiles, filtration, and protective clothing |
| Coaxial electrospinning [81,82,83] | Two or more fluids are spun together in a coaxial arrangement, producing fibers with a core–shell structure | Drug delivery, tissue engineering, and energy storage |
| Multijet electrospinning [83,84,85] | Multiple spinning nozzles are used simultaneously to produce a large volume of fibers in a short amount of time | Tissue engineering, drug delivery, and energy storage |
| Emulsion electrospinning [86,87,88] | An emulsion of two immiscible liquids is spun to create fibers with a polymer and a liquid core | Drug delivery, tissue engineering, and food packaging |
| Methods | Principles | Materials Used | Advantages | Disadvantages |
|---|---|---|---|---|
| Fused deposition modeling [89,90] | Thermoplastic filaments are melted and deposited layer by layer using a heated nozzle | ABS, PLA, Nylon, TPU, etc. | Low cost, user-friendly, wide material compatibility, good for prototyping | Lower resolution, visible layer lines, limited to certain geometries, weaker mechanical properties |
| Stereolithography (SLA) [91,92] | Uses a UV laser to selectively cure a liquid photopolymer resin layer by layer | Resin materials (acrylic, epoxy, polyurethane, etc.) | High resolution, good surface finish, suitable for small and intricate parts, good for prototyping and production | Limited material selection, expensive, post-processing required, not suitable for large parts |
| Selective laser sintering (SLS) [93,94,95] | Uses a laser to selectively fuse a powdered material (such as nylon) layer by layer | Nylon, TPU, polycarbonate, etc. | No support structures required, can produce complex geometries, good mechanical properties, good for low-volume production | Limited surface finish, expensive, post-processing required, not suitable for small parts |
| Selective laser melting [96] | Similar to SLS, but uses a laser to fully melt the powdered material instead of just fusing it, resulting in fully dense metal parts | Metals such as titanium, aluminum, steel, etc. | High strength, good for small and complex parts, wide range of materials available | Expensive, limited build size, slow printing speed, post-processing required |
| Fabrication Methods | Parameters | Considerations |
|---|---|---|
| Electrospinning [119,120,121] | Solution viscosity | Higher viscosity results in larger polymer beads and thicker fibers |
| Solution conductivity | Higher conductivity improves the quality of fibers | |
| Applied voltage | Higher voltage results in thinner fibers | |
| Distance between the collector and the needle | Shorter distance results in denser fibers | |
| Flow rate of the solution | Higher flow rate leads to larger fiber diameter | |
| Needle diameter | Smaller diameter results in thinner fibers | |
| Solution concentration | Higher concentration results in thicker fibers | |
| Humidity and temperature of the environment | Affects fiber morphology and diameter | |
| 3D Printing [89,90,91,92,93,94,95,96] | Material properties | Affects printability and mechanical properties |
| Printing speed | Affects print quality and resolution | |
| Layer height | Affects resolution and mechanical properties | |
| Temperature of the printing environment | Affects material properties and print quality | |
| Extruder speed | Affects flow rate and print quality | |
| Nozzle diameter | Affects resolution and print speed | |
| Printing bed temperature | Affects adhesion and warping | |
| Printing orientation | Affects mechanical properties | |
| Microfluidic method [100,106,107,108,109,110,111] | Flow rate of the fluid | Affects fiber diameter and porosity |
| Viscosity of the fluid | Affects flow rate and fiber morphology | |
| Channel geometry | Affects fiber diameter and alignment | |
| Material properties | Affects fiber morphology and mechanical properties | |
| Surface properties | Affects fiber adhesion | |
| Temperature and pressure of the system | Affects fiber morphology and diameter | |
| Solution blowing [113,114,115] | Solution viscosity | Higher viscosity results in thicker fibers |
| Gas flow rate | Affects fiber diameter and morphology | |
| Distance between the nozzle and the collector | Affects fiber alignment and density | |
| Solution concentration | Higher concentration results in thicker fibers | |
| Temperature and humidity of the environment | Affects fiber morphology and diameter | |
| Centrifugal spinning [116,117,118] | Polymer concentration | Higher concentration results in thicker fibers |
| Centrifugal force | Affects fiber diameter and alignment | |
| Distance between the collector and the nozzle | Affects fiber alignment and density | |
| Solution viscosity | Higher viscosity results in thicker fibers | |
| Solution flow rate | Affects fiber diameter and morphology | |
| Polymer molecular weight | Affects mechanical properties | |
| Temperature and humidity of the environment | Affects fiber morphology and diameter |
| Method | Parameters | Considerations |
|---|---|---|
| Blending [122,123,124,125,126,127] | Solubility of drug and polymer | Drug and polymer should have similar solubilities |
| Drug and polymer concentration | Higher concentrations lead to higher drug loading | |
| Mixing time and speed | Affects drug–polymer interaction and drug distribution | |
| Chemical immobilization [128,129] | Type of chemical reaction | Reaction should be specific to the drug and polymer |
| Concentration of reactants | Higher concentrations lead to higher drug loading | |
| Reaction time and temperature | Affects the efficiency of the reaction and drug loading | |
| Physical adsorption [130,131,132] | Surface area of the scaffold | Larger surface area leads to higher drug loading |
| Solution concentration and pH | Affects drug–polymer interaction and adsorption efficiency | |
| Adsorption time and temperature | Affects the efficiency of drug adsorption | |
| Emulsion electrospinning [133,134,135] | Polymer and drug solubility | Solubility should be matched for codissolving |
| Emulsifier concentration | Affects droplet size and stability | |
| Electrospinning parameters | Affects fiber diameter and drug distribution | |
| Emulsion stability | Emulsion should be stable for efficient drug loading | |
| Coaxial electrospinning [137,138,139] | Core and shell polymer solubility | Solubility should be matched for codissolving |
| Core and shell polymer concentration | Higher concentrations lead to higher drug loading | |
| Coaxial electrospinning parameters | Affects fiber diameter, shell thickness, and drug distribution | |
| Core–shell fiber stability | Core and shell should remain stable during processing | |
| Electrospray [140,141] | Solution concentration and pH | Affects drug–polymer interaction and encapsulation efficiency |
| Flow rate and voltage | Affects droplet size and drug distribution | |
| Solvent type | Affects the solubility of the drug and polymer | |
| Post-processing conditions | Can affect drug loading and release kinetics |
| Biomedical Applications | Fabrication Methods of Scaffold | Therapeutic Agent (with its Function) | Drug-Loading Methods | References |
|---|---|---|---|---|
| Cardiovascular regeneration | Electrospinning method | α-Mangostin (antioxidant, cardioprotective activity) | Dip coating | [144] |
| Cardiovascular regeneration | Electrospinning method with crystallization | Heparin (antithrombosis activity) | Drug-mixing (blending) electrospinning method | [151] |
| Vascular regeneration | Coelectrospinning method | IL-4 (immune modulation for vascular stabilization) | Surface modification of the scaffold | [239] |
| Cardiac regeneration | Electrospinning method | Resveratrol (inducing cardioprotective effect) | Drug-mixing electrospinning method | [240] |
| Nerve regeneration | Electrospinning method | MicroRNAs (miRNAs, genetic modulation for nerve regeneration) | Passive loading after scaffold fabrication | [167] |
| Nerve regeneration | Electrospinning method with rolling | Deferoxamine (promoting angiogenesis with anti-inflammation) | Drug-mixing electrospinning method | [169] |
| Nerve regeneration | Microsol electrospinning | IL-4 encoding plasmid DNA (IL-4 generation for immune suppression) | Chemical modification of pDNA-loaded liposome | [176] |
| Nerve regeneration | Electrospinning method | Magnetic nanoparticles (MgO, inhibiting nerve cell apoptosis), purmorphamine and retinoic acid (Pur/RA, inducing neuronal differentiation) | Pur/RA-loaded MgO-mixing electrospinning method | [241] |
| Nerve regeneration | Electrospinning method | Acidic fibroblast growth factor (aFGF, signaling molecule for axon growth) | Coaxial electrospraying of aFGF-loaded nanoparticles | [242] |
| Nerve regeneration | Microsol electrospinning method | Brain-derived neurotrophic factor (BDNF, improving differentiation of bone marrow mesenchymal stem cells into neurons) | Drug-mixing electrospinning method | [243] |
| Bone regeneration | 3D printing with TIPS technique | Deferoxamine (DFO, inducing angiogenesis for vascularization) | Passive drug loading after scaffold fabrication | [182] |
| Bone regeneration | Coaxial electrospinning method | DFO (inducing vascularization), dexamethasone (DEX, modulating osteogenic differentiation) | Drug-mixing electrospinning method | [15] |
| Bone regeneration | Electrospinning method | Vascular endothelial growth factor (VEGF) encoding pDNA (inducing vascularization), bone morphogenetic protein 2 (BMP2, promoting growth of bone tissue) | Surface immobilization of drug-loaded vector and embedding drug containing hydrogel inside the scaffold | [186] |
| Bone regeneration | Electrospinning method | Tannic acid (TA) and indomethacin (IND) (agents for anti-inflammation) | TA/IND nanomedicine-mixing electrospinning method | [190] |
| Bone regeneration | Coaxial electrospinning method | Tauroursodeoxycholic acid (TUDCA, inducing vascularization), BMP2 (promoting growth of bone tissue) | Drug-mixing electrospinning method | [244] |
| Bone regeneration | Electrospinning method | Recombinant human vein endothelial growth factor (rhVEGF, inducing vascularization), recombinant human bone morphogenetic protein 2 (rhBMP2), and calcium phosphates (inducing bone tissue regeneration) | Drug-mixing electrospinning method | [245] |
| Bone regeneration | Electrospinning method with crosslinking | IL-4 (immunomodulation for vascular maturation and osteogenesis) | Drug-mixing electrospinning method | [246] |
| Wound healing | Electrospinning method | Vitamin K3 carnosine peptide (agent for antibacterial activity) | Drug-mixing electrospinning method | [198] |
| Wound healing | Microsol electrospinning method | IL-10 (agents for anti-inflammatory effect) | Drug-mixing electrospinning method | [210] |
| Wound healing | Electrospinning method | Nitric oxide (NO, agent for antibacterial activity) | Surface modification after scaffold fabrication | [214] |
| Wound healing | Electrospinning method | Cordia myxa (agent for antibacterial activity with antioxidant effect) | Immobilization after scaffold fabrication | [247] |
| Wound healing | Electrospinning method | Anemoside B4 (ANE, agent for anti-inflammation) | Incorporation of ANE after scaffold fabrication | [248] |
| Wound healing | 3D printing | Vascular endothelial growth factor (VEGF, inducing vascularization), gentamicin (antibiotic agent), silver nanoparticle (antibacterial agent) | Drug loading in 3D printing ink | [249] |
| Wound healing | Physical coassembling to construct fibrous hydrogel | Phycocyanin (anti-inflammatory activity), gallic acid (antibacterial activity and anti-inflammatory activity) | Mixing drugs for assembled fibrous scaffold fabrication | [250] |
| Wound healing | Electrospinning method | Manganese dioxide (MnO2, agent for assuaging oxidative stress), curcumin (agent for anti-inflammation) | Curcumin-loaded MnO2 nanoparticle-mixing electrospinning method | [251] |
| Wound healing | Electrospinning method | Pyrogallol and saikosaponin (reducing reactive oxygen species (ROS)) | Drug-mixing electrospinning method | [252] |
| Wound healing | Electrospinning method | Curcumin (agent for anti-inflammation), cerium nitrate (reducing ROS for antiscar wound healing) | Drug-mixing electrospinning method | [253] |
| Wound healing | Layer by layer electrospinning method | Silver (I) sulfadiazine (agent for antibacterial activity) | Drug-mixing electrospinning method | [254] |
| Tumor inhibition | 3D printing with electrospinning method for multiple-layered scaffold fabrication | Combretastin A4 (CA4, inhibiting tumor growth) and doxorubicin (DOX, inhibiting metastasis and recurrence of cancer) | DOX: drug-mixing electrospinning method, CA4: drug-mixing in bioink before 3D printing | [225] |
| Tumor inhibition with antibacterial activity | Electrospinning method | Gemicitabine (GEM, inhibits tumor growth), silver nanoparticle (antibacterial agent for enhancing tumor therapy) | GEM: drug mixing electrospinning method, silver nanoparticle: dipping process after scaffold fabrication with coating | [228] |
| Tumor inhibition | Sol–gel process with electrospinning method | Glucose oxidase (GOx, inducing starvation activity around tumor tissue), hyaluronidase (HAase, destroying tumor extracellular matrix (ECM) to penetrate nanomedicine), banoxantrone (AQ4N, antitumor agent) | Incorporation of HAase and GOx/AQ4N-loaded nanoparticles after scaffold fabrication | [255] |
| Tumor inhibition | Coaxial 3D printing | DOX (antitumor agent), polydopamine (photothermal agent for thermal-induced tumor therapy) | Mixing drugs in ink and fabricating scaffold | [256] |
| Tumor inhibition with wound healing | Coaxial electrospinning method | Fluorouracil (5-FU, agent for chemotherapy of melanoma) | 5-FU-loaded nanoparticle-mixing electrospinning method | [257] |
| Periodontal regeneration | Electrospinning method | Dimethyloxalylglycine (DMOG, inhibitor of prolyl hydroxylases to promote VEGF) | Drug-mixing electrospinning method | [231] |
| Tendon regeneration | Electrospinning method | Nitric oxide (NO, agent for vascularization) | Use of NO-loaded metal–organic framework-mixing electrospinning method | [237] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.; Lee, S.J.; Choi, D.K.; Park, J.-W. Therapeutic Agent-Loaded Fibrous Scaffolds for Biomedical Applications. Pharmaceutics 2023, 15, 1522. https://doi.org/10.3390/pharmaceutics15051522
Park D, Lee SJ, Choi DK, Park J-W. Therapeutic Agent-Loaded Fibrous Scaffolds for Biomedical Applications. Pharmaceutics. 2023; 15(5):1522. https://doi.org/10.3390/pharmaceutics15051522
Chicago/Turabian StylePark, Dongsik, Su Jin Lee, Dong Kyu Choi, and Jee-Woong Park. 2023. "Therapeutic Agent-Loaded Fibrous Scaffolds for Biomedical Applications" Pharmaceutics 15, no. 5: 1522. https://doi.org/10.3390/pharmaceutics15051522
APA StylePark, D., Lee, S. J., Choi, D. K., & Park, J. -W. (2023). Therapeutic Agent-Loaded Fibrous Scaffolds for Biomedical Applications. Pharmaceutics, 15(5), 1522. https://doi.org/10.3390/pharmaceutics15051522