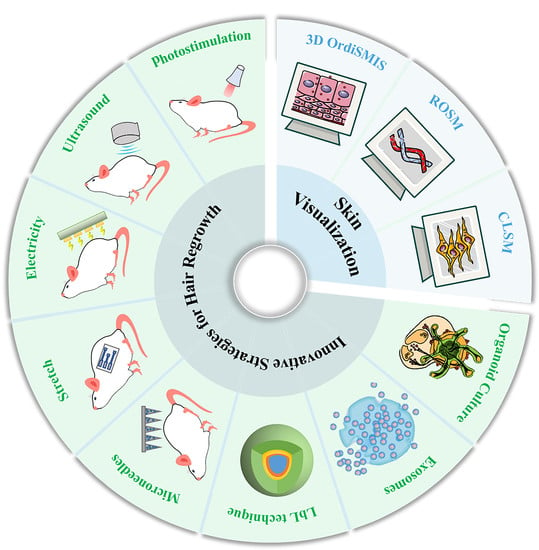
Innovative Strategies for Hair Regrowth and Skin Visualization
Abstract
:1. Introduction
2. Structure of the Skin and Hair Follicles
3. Molecular Mechanisms of Hair Regrowth
4. Innovative Strategies for Hair Regrowth
4.1. External Stimulation
4.1.1. Photostimulation
4.1.2. Ultrasound Stimulation
4.1.3. Electrical Stimulation
4.1.4. Stretch Stimulation
4.2. Microneedles for Hair Regrowth
4.3. Regenerative Medicine for Hair Regrowth
4.3.1. Biomimetic Extracellular Matrix
4.3.2. Extracellular Vesicles
4.3.3. Organoid Culture
5. Skin Visualization
6. Claims for Hair Regrowth
7. Natural Products for Hair Regrowth
8. Patent Applications and Clinical Trials for Hair Loss Treatment
| Classification | Patent/Application Number | Patent Title | Features | Assignee | Filling Year | Status |
|---|---|---|---|---|---|---|
| Equipment | CN201711166676.8 [114] | Application of LED-red light with 610 nm–650 nm wavelengths in alopecia treatment | A 610 nm and 650 nm LED red light can effectively prevent hair loss | Harbin Medical University | 2018 | Granted |
| CN202010582452.0 [115] | Equipment for laser therapy on androgenetic alopecia and alopecia treatment methods | The laser sterilizes the area of hair loss | Liruiya | 2021 | Granted | |
| Regenerative medicine | CN202210069596.5 [113] | A method of HHORSCs exosomes promoting dermal papilla cells to induce hair regeneration | Promotes DPCs to induce hair regrowth | Guangdong LIYI Technology Co. Ltd. | 2022 | Granted |
| US17693457 [116] | Methods and compositions for aesthetic and cosmetic treatment and stimulating hair growth | Increases skin volume to prevent or treat hair loss and related diseases | Pluristem Ltd. | 2019 | Granted | |
| PCT/IB2021/062226 [117] | Use of miRNA-485 inhibitors for inducing hair regrowth | Increases hair density, follicle density, and hair shaft thickness | Biorchestra Co. Ltd. | 2022 | Granted | |
| Drug | US17562976 [118] | Hydrazone amide derivative and application thereof in the preparation of medicaments for preventing and treating alopecia | \ | Shenzhen Cell Inspire Pharmaceutical Dev Co. Ltd. | 2022 | Granted |
| Study Title | Medication | ClinicalTrials.gov Identifier | Status | Study Date |
|---|---|---|---|---|
| ENERGI-F701 for Female Hair Loss Treatment | Drug: ENERGI-F701 | NCT03351322 | Phase 2 | May 2018–December 2019 |
| Safety and Efficacy of HST 001 in Male Pattern Hair Loss | Biological: HST 001–0.1 mL X 20 injections | NCT04435847 | Phase 1 | May 2020–January 2021 |
| Modulated Light Therapy in Participants With Pattern Hair Loss | Device: REVIAN 101 Device: REVIAN 102 Device: REVIAN 103 Device: REVIAN 100 | NCT04019795 | Phase 3 | January 2017–May 2019 |
| Adipose-derived Stem Cell Conditioned Media as a Novel Approach for Hair Regrowth in Male Androgenetic Alopecia | Combination Product: Non-concentrated adipose-derived stem cell conditioned media and 5% Minoxidil Combination Product: Concentrated adipose-derived stem cell conditioned media and 5% Minoxidil Combination Product: Placebo and 5% Minoxidil | NCT05296863 | Phase 3 | October 2021– December 2021 |
| Androgenetic Alopecia Treatment Using Varin and Cannabidiol Rich Topical Hemp Oil: A Case Series (Hair Regeneration) | Drug: Hemp Oil | NCT04842383 | Early Phase 1 | April, 2021–October 2021 |
| Topical Cetirizine in Androgenetic Alopecia in Females | Topical cetirizine | NCT04481412 | Phase 2 Phase 3 | Ongoing since July 2020 |
| A Study Evaluating the Efficacy and Safety of SM04554 Topical Solution in Male Subjects With Androgenetic Alopecia | Topical SM04554 solution | NCT03742518 | Phase 2 Phase 3 | November 2018–December 2021 |
| Safety, Tolerability and Pharmacokinetics of KX826 in Healthy Male Subjects With Androgenetic Alopecia Following Topical Single Ascending Dose Administration | KX0826 | NCT04984707 | Phase 1 | January 2019–October 2019 |
| Safety and Efficacy Study of Topical DLQ01 in the Treatment of Androgenetic Alopecia (AGA) in Men | Drug: prostaglandin F2a analogue in vehicle solution high dose Drug: prostaglandin F2a analogue in vehicle solution low dose Drug: active ingredient-free vehicle solution to DLQ01 | NCT05636904 | Phase 1 Phase 2 | December 2022–March 2024 |
| Topical Cetirizine 1% vs Minoxidil 5% Gel in Treatment of Androgenetic Alopecia | Drug: Cetirizine Drug: Minoxidil | NCT04293822 | Phase 4 | June 2020–November 2021 |
9. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Islam, N.; Leung, P.S.; Huntley, A.C.; Gershwin, M.E. The autoimmune basis of alopecia areata: A comprehensive review. Autoimmun. Rev. 2015, 14, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Perper, M.; Aldahan, A.S.; Fayne, R.A.; Emerson, C.P.; Nouri, K. Efficacy of fractional lasers in treating alopecia: A literature review. Lasers Med. Sci. 2017, 32, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.; Gonzalez, M.; Finlay, A.Y. The effect of hair loss on quality of life. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.X.; Sidorenko, J.; Wu, Y.; Kemper, K.E.; Yang, J.; Wray, N.R.; Robinson, M.R.; Visscher, P.M. Dissection of genetic variation and evidence for pleiotropy in male pattern baldness. Nat. Commun. 2018, 9, 5407. [Google Scholar] [CrossRef] [Green Version]
- Heilmann-Heimbach, S.; Herold, C.; Hochfeld, L.M.; Hillmer, A.M.; Nyholt, D.R.; Hecker, J.; Javed, A.; Chew, E.G.; Pechlivanis, S.; Drichel, D.; et al. Meta-analysis identifies novel risk loci and yields systematic insights into the biology of male-pattern baldness. Nat. Commun. 2017, 8, 14694. [Google Scholar] [CrossRef] [Green Version]
- Randall, V.A.; Thornton, M.J.; Hamada, K.; Messenger, A.G. Mechanism of androgen action in cultured dermal papilla cells derived from human hair follicles with varying responses to androgens in vivo. J. Investig. Dermatol. 1992, 98, 86S–91S. [Google Scholar] [CrossRef] [Green Version]
- Kwack, M.H.; Ahn, J.S.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dihydrotestosterone-inducible IL-6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice. J. Investig. Dermatol. 2012, 132, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Phan, K.; Sebaratnam, D.F. JAK inhibitors for alopecia areata: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 850–856. [Google Scholar] [CrossRef]
- Yang, G.; Chen, Q.; Wen, D.; Chen, Z.; Wang, J.; Chen, G.; Wang, Z.; Zhang, X.; Zhang, Y.; Hu, Q.; et al. A Therapeutic Microneedle Patch Made from Hair-Derived Keratin for Promoting Hair Regrowth. ACS Nano 2019, 13, 4354–4360. [Google Scholar] [CrossRef]
- Randolph, M.; Tosti, A. Oral minoxidil treatment for hair loss: A review of efficacy and safety. J. Am. Acad. Dermatol. 2021, 84, 737–746. [Google Scholar] [CrossRef]
- Gupta, A.K.; Talukder, M.; Venkataraman, M.; Bamimore, M.A. Minoxidil: A comprehensive review. J. Dermatol. Treat. 2022, 33, 1896–1906. [Google Scholar] [CrossRef]
- Kochar, P.; Nayak, K.; Thakkar, S.; Polaka, S.; Khunt, D.; Misra, M. Exploring the potential of minoxidil tretinoin liposomal based hydrogel for topical delivery in the treatment of androgenic alopecia. Cutan. Ocul. Toxicol. 2020, 39, 43–53. [Google Scholar] [CrossRef]
- Ramkar, S.; Kaurav, M.; Sudheesh, M.S.; Pandey, R.S. Enhanced skin penetration of Finasteride loaded DMSO-liposomes for the treatment of androgenic alopecia: Comparison with conventional liposomes. Drug Dev. Ind. Pharm. 2023, 49, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, Y.; Wang, M.; Yang, X.; Tang, Y.; Pang, M.; Wang, W.; Chen, L.; Wu, C.; Xu, Y. Microneedles mediated bioinspired lipid nanocarriers for targeted treatment of alopecia. J. Control. Release 2021, 329, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Jee, S.H.; Chan, J.L. Hair transplantation for the treatment of lichen planopilaris and frontal fibrosing alopecia: A report of two cases. Australas. J. Dermatol. 2018, 59, e118–e122. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Cotsarelis, G. Gene expression profiling gets to the root of human hair follicle stem cells. J. Clin. Investig. 2006, 116, 19–22. [Google Scholar] [CrossRef]
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.C.; Rose, C.; Yoon, G.S.; Lee, S.J.; et al. A Guide to Studying Human Hair Follicle Cycling In Vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [Green Version]
- Blanpain, C.; Fuchs, E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Liu, X.; Qu, Q.; Chen, J.; Fan, Z.; Zhu, D.; Miao, Y.; Hu, Z. Overexpression of miR-122 promotes apoptosis of dermal papilla cells by directly targeting IGF1R in androgenetic alopecia. Cell Biol. Int. 2022, 46, 185–191. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Peng, Y.; Zhang, L.; Yu, Y.; Hua, P.; Zhu, P.; Yan, X.; Li, Y.; Zhang, L. miR-24 controls the regenerative competence of hair follicle progenitors by targeting Plk3. Cell Rep. 2021, 35, 109225. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Lutz, H.; Huang, K.; Su, T.; Cores, J.; Dinh, P.C.; Cheng, K. Dermal exosomes containing miR-218-5p promote hair regeneration by regulating beta-catenin signaling. Sci. Adv. 2020, 6, eaba1685. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guerrero-Juarez, C.F.; Xiao, F.; Shettigar, N.U.; Ramos, R.; Kuan, C.H.; Lin, Y.C.; de Jesus Martinez Lomeli, L.; Park, J.M.; Oh, J.W.; et al. Hedgehog signaling reprograms hair follicle niche fibroblasts to a hyper-activated state. Dev. Cell 2022, 57, 1758–1775. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Ding, X.; Allmeroth, K.; Biggs, L.C.; Kolenc, O.I.; L’Hoest, N.; Chacon-Martinez, C.A.; Edlich-Muth, C.; Giavalisco, P.; Quinn, K.P.; et al. Glutamine Metabolism Controls Stem Cell Fate Reversibility and Long-Term Maintenance in the Hair Follicle. Cell Metab. 2020, 32, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, X.; Liang, Y.; Yu, J.; Li, H.; Shokhirev, M.N.; Zheng, Y. Glucocorticoid signaling and regulatory T cells cooperate to maintain the hair-follicle stem-cell niche. Nat. Immunol. 2022, 23, 1086–1097. [Google Scholar] [CrossRef]
- Choi, S.; Zhang, B.; Ma, S.; Gonzalez-Celeiro, M.; Stein, D.; Jin, X.; Kim, S.T.; Kang, Y.L.; Besnard, A.; Rezza, A.; et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 2021, 592, 428–432. [Google Scholar] [CrossRef]
- Ali, N.; Zirak, B.; Rodriguez, R.S.; Pauli, M.L.; Truong, H.A.; Lai, K.; Ahn, R.; Corbin, K.; Lowe, M.M.; Scharschmidt, T.C.; et al. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 2017, 169, 1119–1129. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Tang, X.; Zhang, S.; Jin, M.; Wang, M.; Deng, Z.; Liu, Z.; Qian, M.; Shi, W.; Wang, Z.; et al. SIRT7 activates quiescent hair follicle stem cells to ensure hair growth in mice. EMBO J. 2020, 39, e104365. [Google Scholar] [CrossRef]
- Jin, H.; Zou, Z.; Chang, H.; Shen, Q.; Liu, L.; Xing, D. Photobiomodulation therapy for hair regeneration: A synergetic activation of beta-CATENIN in hair follicle stem cells by ROS and paracrine WNTs. Stem Cell Rep. 2021, 16, 1568–1583. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Jiang, D.; Li, J.; Kang, L.; Chen, S.; Long, Y.; Wang, Y.; Huang, P.; Lin, Y.; Cai, W.; et al. Self-Activated Electrical Stimulation for Effective Hair Regeneration via a Wearable Omnidirectional Pulse Generator. ACS Nano 2019, 13, 12345–12356. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.Y.; Chou, C.H.; Huang, H.D.; Yen, M.H.; Hong, H.C.; Chao, P.H.; Wang, Y.H.; Chen, P.Y.; Nian, S.X.; Chen, Y.R.; et al. Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat. Commun. 2019, 10, 1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodarzi, H.R.; Abbasi, A.; Saffari, M.; Tabei, M.B.; Noori Daloii, M.R. MicroRNAs take part in pathophysiology and pathogenesis of Male Pattern Baldness. Mol. Biol. Rep. 2010, 37, 2959–2965. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 2013, 34, 518–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco, E.; Calvo, M.I.; Blazquez-Castro, A.; Vecchio, D.; Zamarron, A.; de Almeida, I.J.D.; Stockert, J.C.; Hamblin, M.R.; Juarranz, A.; Espada, J. Photoactivation of ROS Production In Situ Transiently Activates Cell Proliferation in Mouse Skin and in the Hair Follicle Stem Cell Niche Promoting Hair Growth and Wound Healing. J. Investig. Dermatol. 2015, 135, 2611–2622. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.E.; Lee, S.H.; Jeong, M.; Shin, J.H.; Ahn, Y.; Kim, D.; Oh, S.H.; Yun, S.H.; Lee, K.J. Trichogenic Photostimulation Using Monolithic Flexible Vertical AlGaInP Light-Emitting Diodes. ACS Nano 2018, 12, 9587–9595. [Google Scholar] [CrossRef]
- Yang, K.; Li, D.; Wang, M.; Xu, Z.; Chen, X.; Liu, Q.; Sun, W.; Li, J.; Gong, Y.; Liu, D.; et al. Exposure to blue light stimulates the proangiogenic capability of exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2019, 10, 358. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Won, E.J.; Lee, H.A.R.; Kim, J.H.; Hui, E.; Kim, H.P.; Yoon, T.J. Ultrasound-activated particles as CRISPR/Cas9 delivery system for androgenic alopecia therapy. Biomaterials 2020, 232, 119736. [Google Scholar] [CrossRef]
- Adaikkan, C.; Middleton, S.J.; Marco, A.; Pao, P.C.; Mathys, H.; Kim, D.N.; Gao, F.; Young, J.Z.; Suk, H.J.; Boyden, E.S.; et al. Gamma Entrainment Binds Higher-Order Brain Regions and Offers Neuroprotection. Neuron 2019, 102, 929–943. [Google Scholar] [CrossRef]
- Lim, C.; Hong, Y.J.; Jung, J.; Shin, Y.; Sunwoo, S.H.; Baik, S.; Park, O.K.; Choi, S.H.; Hyeon, T.; Kim, J.H.; et al. Tissue-like skin-device interface for wearable bioelectronics by using ultrasoft, mass-permeable, and low-impedance hydrogels. Sci. Adv. 2021, 7, eabd3716. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Sifrim, A.; Malfait, M.; Song, Y.; Van Herck, J.; Dekoninck, S.; Gargouri, S.; Lapouge, G.; Swedlund, B.; Dubois, C.; et al. Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature 2020, 584, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Mester, E.; Szende, B.; Gartner, P. The effect of laser beams on the growth of hair in mice. Radiobiol. Radiother. 1968, 9, 621–626. [Google Scholar]
- Szezerbaty, S.K.F.; de Oliveira, R.F.; Pires-Oliveira, D.A.A.; Soares, C.P.; Sartori, D.; Poli-Frederico, R.C. The effect of low-level laser therapy (660 nm) on the gene expression involved in tissue repair. Lasers Med. Sci. 2018, 33, 315–321. [Google Scholar] [CrossRef]
- Shen, S.; Fan, D.; Yuan, Y.; Ma, X.; Zhao, J.; Yang, J. An ultrasmall infinite coordination polymer nanomedicine-composited biomimetic hydrogel for programmed dressing-chemo-low level laser combination therapy of burn wounds. Chem. Eng. J. 2021, 426, 130610. [Google Scholar] [CrossRef]
- Beauvoit, B.; Kitai, T.; Chance, B. Contribution of the mitochondrial compartment to the optical properties of the rat liver: A theoretical and practical approach. Biophys. J. 1994, 67, 2501–2510. [Google Scholar] [CrossRef] [Green Version]
- Pastore, D.; Greco, M.; Passarella, S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int. J. Radiat. Biol. 2000, 76, 863–870. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: Role of intracellular calcium and light-gated ion channels. Sci. Rep. 2016, 6, 33719. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.H.; Bae, M.H.; Kim, D.G.; Cheng, H.; Kim, B.H.; Kim, D.H.; Li, M.; Wu, J.; Du, F.; Kim, H.S.; et al. Stretchable, transparent graphene interconnects for arrays of microscale inorganic light emitting diodes on rubber substrates. Nano Lett. 2011, 11, 3881–3886. [Google Scholar] [CrossRef]
- Kim, R.H.; Kim, D.H.; Xiao, J.; Kim, B.H.; Park, S.I.; Panilaitis, B.; Ghaffari, R.; Yao, J.; Li, M.; Liu, Z.; et al. Waterproof AlInGaP optoelectronics on stretchable substrates with applications in biomedicine and robotics. Nat. Mater. 2010, 9, 929–937. [Google Scholar] [CrossRef]
- Kim, R.H.; Tao, H.; Kim, T.I.; Zhang, Y.; Kim, S.; Panilaitis, B.; Yang, M.; Kim, D.H.; Jung, Y.H.; Kim, B.H.; et al. Materials and designs for wirelessly powered implantable light-emitting systems. Small 2012, 8, 2812–2818. [Google Scholar] [CrossRef]
- Lee, J.M.; Choung, J.W.; Yi, J.; Lee, D.H.; Samal, M.; Yi, D.K.; Lee, C.H.; Yi, G.C.; Paik, U.; Rogers, J.A.; et al. Vertical pillar-superlattice array and graphene hybrid light emitting diodes. Nano Lett. 2010, 10, 2783–2788. [Google Scholar] [CrossRef]
- Park, S.I.; Le, A.P.; Wu, J.; Huang, Y.; Li, X.; Rogers, J.A. Light emission characteristics and mechanics of foldable inorganic light-emitting diodes. Adv. Mater. 2010, 22, 3062–3066. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.M.; Chang, Y.T.; Chen, C.L.; Wang, W.H.; Pan, M.K.; Chen, W.P.; Huang, W.Y.; Xu, Z.; Huang, H.E.; Chen, T.; et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc. Natl. Acad. Sci. USA 2018, 115, E6880–E6889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boissenot, T.; Bordat, A.; Fattal, E.; Tsapis, N. Ultrasound-triggered drug delivery for cancer treatment using drug delivery systems: From theoretical considerations to practical applications. J. Control. Release 2016, 241, 144–163. [Google Scholar] [CrossRef]
- Snipstad, S.; Sulheim, E.; de Lange Davies, C.; Moonen, C.; Storm, G.; Kiessling, F.; Schmid, R.; Lammers, T. Sonopermeation to improve drug delivery to tumors: From fundamental understanding to clinical translation. Expert Opin. Drug Deliv. 2018, 15, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Azagury, A.; Khoury, L.; Enden, G.; Kost, J. Ultrasound mediated transdermal drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 127–143. [Google Scholar] [CrossRef]
- Liao, A.H.; Huang, Y.J.; Chuang, H.C.; Wang, C.H.; Shih, C.P.; Chiang, C.P. Minoxidil-Coated Lysozyme-Shelled Microbubbes Combined With Ultrasound for the Enhancement of Hair Follicle Growth: Efficacy In Vitro and In Vivo. Front. Pharmacol. 2021, 12, 668754. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, T.; Han, L.; Zhu, M.; Cheng, Z.; Li, D.; Ren, F.; Wang, K.; Lu, X. Conductive Cellulose Bio-Nanosheets Assembled Biostable Hydrogel for Reliable Bioelectronics. Adv. Funct. Mater. 2021, 31, 2010465. [Google Scholar] [CrossRef]
- Yu, W.S.; Tse, A.C.; Guan, L.; Chiu, J.L.Y.; Tan, S.Z.K.; Khairuddin, S.; Agadagba, S.K.; Lo, A.C.Y.; Fung, M.L.; Chan, Y.S.; et al. Antidepressant-like effects of transcorneal electrical stimulation in rat models. Brain Stimul. 2022, 15, 843–856. [Google Scholar] [CrossRef]
- Liu, A.; Long, Y.; Li, J.; Gu, L.; Karim, A.; Wang, X.; Gibson, A.L.F. Accelerated complete human skin architecture restoration after wounding by nanogenerator-driven electrostimulation. J. Nanobiotechnol. 2021, 19, 280. [Google Scholar] [CrossRef]
- Luo, R.; Dai, J.; Zhang, J.; Li, Z. Accelerated Skin Wound Healing by Electrical Stimulation. Adv. Healthc. Mater. 2021, 10, e2100557. [Google Scholar] [CrossRef]
- Benjamin, B.; Ziginskas, D.; Harman, J.; Meakin, T. Pulsed electrostatic fields (ETG) to reduce hair loss in women undergoing chemotherapy for breast carcinoma: A pilot study. Psychooncology 2002, 11, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Bureau, J.P.; Ginouves, P.; Guilbaud, J.; Roux, M.E. Essential oils and low-intensity electromagnetic pulses in the treatment of androgen-dependent alopecia. Adv. Ther. 2003, 20, 220–229. [Google Scholar] [CrossRef]
- Yan, L.; Kageyama, T.; Zhang, B.; Yamashita, S.; Molino, P.J.; Wallace, G.G.; Fukuda, J. Electrical stimulation to human dermal papilla cells for hair regenerative medicine. J. Biosci. Bioeng. 2022, 133, 281–290. [Google Scholar] [CrossRef]
- Sohn, K.M.; Jeong, K.H.; Kim, J.E.; Park, Y.M.; Kang, H. Hair growth-promotion effects of different alternating current parameter settings are mediated by the activation of Wnt/beta-catenin and MAPK pathway. Exp. Dermatol. 2015, 24, 958–963. [Google Scholar] [CrossRef]
- Hwang, D.; Lee, H.; Lee, J.; Lee, M.; Cho, S.; Kim, T.; Kim, H. Micro-Current Stimulation Has Potential Effects of Hair Growth-Promotion on Human Hair Follicle-Derived Papilla Cells and Animal Model. Int. J. Mol. Sci. 2021, 22, 4361. [Google Scholar] [CrossRef]
- Shi, N.; Li, Y.; Chang, L.; Zhao, G.; Jin, G.; Lyu, Y.; Genin, G.M.; Ma, Y.; Xu, F. A 3D, Magnetically Actuated, Aligned Collagen Fiber Hydrogel Platform Recapitulates Physical Microenvironment of Myoblasts for Enhancing Myogenesis. Small Methods 2021, 5, e2100276. [Google Scholar] [CrossRef] [PubMed]
- Fakhraei Lahiji, S.; Seo, S.H.; Kim, S.; Dangol, M.; Shim, J.; Li, C.G.; Ma, Y.; Lee, C.; Kang, G.; Yang, H.; et al. Transcutaneous implantation of valproic acid-encapsulated dissolving microneedles induces hair regrowth. Biomaterials 2018, 167, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Xia, F.; Bian, Q.; Wu, H.; Gu, Y.; Wang, T.; Wang, R.; Huang, L.; Huang, Q.; Rao, Y.; et al. Ceria Nanozyme-Integrated Microneedles Reshape the Perifollicular Microenvironment for Androgenetic Alopecia Treatment. ACS Nano 2021, 15, 13759–13769. [Google Scholar] [CrossRef]
- English, R.S., Jr.; Ruiz, S.; DoAmaral, P. Microneedling and Its Use in Hair Loss Disorders: A Systematic Review. Dermatol. Ther. 2022, 12, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Miao, Y.; Zhang, F.; Huang, J.; Chen, Y.; Fan, Z.; Yang, L.; Wang, J.; Hu, Z. Nanoscale microenvironment engineering based on layer-by-layer self-assembly to regulate hair follicle stem cell fate for regenerative medicine. Theranostics 2020, 10, 11673–11689. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Miao, Y.; Huang, Y.; Lin, B.; Liu, X.; Xiao, S.; Du, L.; Hu, Z.; Xing, M. Bottom-up Nanoencapsulation from Single Cells to Tunable and Scalable Cellular Spheroids for Hair Follicle Regeneration. Adv. Healthc. Mater. 2018, 7, 1700447. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Miao, Y.; Zhang, F.; Fan, Z.; Huang, J.; Mao, X.; Chen, J.; Hu, Z.; Wang, J. Tissue engineering ECM-enriched controllable vascularized human microtissue for hair regenerative medicine using a biomimetic developmental approach. J. Adv. Res. 2022, 38, 77–89. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, J.; Chen, R.; Yang, L.; Wang, J.; Liu, B.; Du, L.; Yi, Y.; Jia, J.; Xu, Y.; et al. Sustained release of dermal papilla-derived extracellular vesicles from injectable microgel promotes hair growth. Theranostics 2020, 10, 1454–1478. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, M.J.; Lee, Y.J.; Lee, J.C.; Kim, J.H.; Kim, D.H.; Do, Y.H.; Choi, J.W.; Chung, S.I.; Do, B.R. Innovative method of alopecia treatment by autologous adipose-derived SVF. Stem Cell Res. Ther. 2021, 12, 486. [Google Scholar] [CrossRef]
- Lu, Q.; Gao, Y.; Fan, Z.; Xiao, X.; Chen, Y.; Si, Y.; Kong, D.; Wang, S.; Liao, M.; Chen, X.; et al. Amphiregulin promotes hair regeneration of skin-derived precursors via the PI3K and MAPK pathways. Cell Prolif. 2021, 54, e13106. [Google Scholar] [CrossRef]
- Lee, J.; van der Valk, W.H.; Serdy, S.A.; Deakin, C.; Kim, J.; Le, A.P.; Koehler, K.R. Generation and characterization of hair-bearing skin organoids from human pluripotent stem cells. Nat. Protoc. 2022, 17, 1266–1305. [Google Scholar] [CrossRef]
- Toyoshima, K.E.; Ogawa, M.; Tsuji, T. Regeneration of a bioengineered 3D integumentary organ system from iPS cells. Nat. Protoc. 2019, 14, 1323–1338. [Google Scholar] [CrossRef]
- Lee, J.; Rabbani, C.C.; Gao, H.; Steinhart, M.R.; Woodruff, B.M.; Pflum, Z.E.; Kim, A.; Heller, S.; Liu, Y.; Shipchandler, T.Z.; et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 2020, 582, 399–404. [Google Scholar] [CrossRef]
- Ha, L.; Hundt, J.E. Optical coherence tomography for fast bedside imaging, assessment and monitoring of autoimmune inflammatory skin diseases? J. Dtsch. Dermatol. Ges. 2020, 18, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, J.; Brown, C.M.; Wright, G.D.; Anderson, K.I.; North, A.J. Tutorial: Guidance for quantitative confocal microscopy. Nat. Protoc. 2020, 15, 1585–1611. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.H.; Kim, K.H.; Chung, K.Y. Skin Imaging Using Ultrasound Imaging, Optical Coherence Tomography, Confocal Microscopy, and Two-Photon Microscopy in Cutaneous Oncology. Front. Med. 2019, 6, 274. [Google Scholar] [CrossRef]
- He, M.J.; Pu, W.; Wang, X.; Zhang, W.; Tang, D.; Dai, Y. Comparing DESI-MSI and MALDI-MSI Mediated Spatial Metabolomics and Their Applications in Cancer Studies. Front. Oncol. 2022, 12, 891018. [Google Scholar] [CrossRef] [PubMed]
- Quartier, J.; Rao, W.; Slade, S.; Metral, F.; Lapteva, M.; Kalia, Y.N. DESI-MS imaging to visualize spatial distribution of xenobiotics and endogenous lipids in the skin. Int. J. Pharm. 2021, 607, 120967. [Google Scholar] [CrossRef]
- Hindelang, B.; Aguirre, J.; Schwarz, M.; Berezhnoi, A.; Eyerich, K.; Ntziachristos, V.; Biedermann, T.; Darsow, U. Non-invasive imaging in dermatology and the unique potential of raster-scan optoacoustic mesoscopy. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1051–1061. [Google Scholar] [CrossRef] [Green Version]
- Starr, N.J.; Khan, M.H.; Edney, M.K.; Trindade, G.F.; Kern, S.; Pirkl, A.; Kleine-Boymann, M.; Elms, C.; O’Mahony, M.M.; Bell, M.; et al. Elucidating the molecular landscape of the stratum corneum. Proc. Natl. Acad. Sci. USA 2022, 119, e2114380119. [Google Scholar] [CrossRef]
- Nitkunanantharajah, S.; Zahnd, G.; Olivo, M.; Navab, N.; Mohajerani, P.; Ntziachristos, V. Skin Surface Detection in 3D Optoacoustic Mesoscopy Based on Dynamic Programming. IEEE Trans. Med. Imaging 2020, 39, 458–467. [Google Scholar] [CrossRef] [Green Version]
- Herman, A.; Herman, A.P. Topically used herbal products for the treatment of hair loss: Preclinical and clinical studies. Arch Dermatol. Res. 2017, 309, 595–610. [Google Scholar] [CrossRef]
- Yuen, G.K.; Ho, B.S.; Lin, L.S.; Dong, T.T.; Tsim, K.W. Tectoridin Stimulates the Activity of Human Dermal Papilla Cells and Promotes Hair Shaft Elongation in Mouse Vibrissae Hair Follicle Culture. Molecules 2022, 27, 400. [Google Scholar] [CrossRef]
- Iwabuchi, T.; Ogura, K.; Tamba, K.; Tsunekawa, Y.; Sugano, M.; Hagiwara, K.; Kiso, A. Cepharanthine induces the proliferation of human dermal papilla cells and stimulates vascular endothelial growth factor expression through increased intracellular calcium mobilization and hypoxia-inducible factor activation. Clin. Exp. Dermatol. 2021, 46, 694–703. [Google Scholar] [CrossRef]
- Morita, K.; Nakamura, M.; Nagamachi, M.; Kishi, T.; Miyachi, Y. Seventeen cases of alopecia areata: Combination of SADBE topical immunotherapy with other therapies. J. Dermatol. 2002, 29, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, S.H.; Yang, W.M. Beneficial effects of Astragaloside IV for hair loss via inhibition of Fas/Fas L-mediated apoptotic signaling. PLoS ONE 2014, 9, e92984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.C.; Kang, J.I.; Park, D.B.; Lee, Y.K.; Hyun, J.W.; Koh, Y.S.; Yoo, E.S.; Kim, J.A.; Kim, Y.H.; Kang, H.K. Promotion effect of acankoreoside J, a lupane-triterpene in Acanthopanax koreanum, on hair growth. Arch Pharm. Res. 2012, 35, 1495–1503. [Google Scholar] [CrossRef]
- Kim, Y.E.; Choi, H.C.; Lee, I.-C.; Yuk, D.Y.; Lee, H.; Choi, B.Y. 3-Deoxysappanchalcone Promotes Proliferation of Human Hair Follicle Dermal Papilla Cells and Hair Growth in C57BL/6 Mice by Modulating WNT/β-Catenin and STAT Signaling. Biomol. Ther. 2016, 24, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Han, L.; Chen, S.S.; Guan, J.; Qu, F.Z.; Zhao, Y.Q. Hair growth promoting activity of cedrol isolated from the leaves of Platycladus orientalis. Biomed. Pharmacother. 2016, 83, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Zhang, Y.; Lu, Q.L.; Lin, Z.; Zhao, Y. Preventive effects of cedrol against alopecia in cyclophosphamide-treated mice. Environ. Toxicol. Pharmacol. 2016, 46, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Huang, F.; Wang, J.; Zhang, Y.; Zhang, Y.; Su, G.; Zhao, Y. Hair Growth Promoting Activity of Cedrol Nanoemulsion in C57BL/6 Mice and Its Bioavailability. Molecules 2021, 26, 1795. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.W.; Qu, F.Z.; Zhang, Y.M.; Su, G.Y.; Zhao, Y.Q. Hair growth promotion effect of cedrol cream and its dermatopharmacokinetics. RSC Adv. 2018, 8, 42170–42178. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.H.; Bak, S.S.; Kim, M.K.; Sung, Y.K.; Kim, J.C. Baicalin, a flavonoid, affects the activity of human dermal papilla cells and promotes anagen induction in mice. Naunyn. Schmiedebergs Arch Pharmacol. 2015, 388, 583–586. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, Z.; Zhu, Z.; Hu, Y.; Wang, Y.; Xue, Y.; Wu, Y.; Guo, Y.; Liang, P.; Chen, H.; et al. Glycyrrhizin micellar nanocarriers for topical delivery of baicalin to the hair follicles: A targeted approach tailored for alopecia treatment. Int. J. Pharm. 2022, 625, 122109. [Google Scholar] [CrossRef] [PubMed]
- Lao, Z.; Fan, Y.; Huo, Y.; Liao, F.; Zhang, R.; Zhang, B.; Kong, Z.; Long, H.; Xie, J.; Sang, C.; et al. Physcion, a novel inhibitor of 5alpha-reductase that promotes hair growth in vitro and in vivo. Arch Dermatol. Res. 2022, 314, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.R.; Choi, Y.H.; Shin, J.Y.; Kim, C.D.; Kang, N.G.; Park, B.C.; Lee, S. Quercitrin Stimulates Hair Growth with Enhanced Expression of Growth Factors via Activation of MAPK/CREB Signaling Pathway. Molecules 2020, 25, 4004. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Villasante, A.C.; Mauro, L.M.; Perez, C.I.; Schachner, L.A.; Jimenez, J.J. Prevention and treatment of alopecia areata with quercetin in the C3H/HeJ mouse model. Cell Stress Chaperones 2012, 17, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Das, L.; Kaurav, M.; Pandey, R.S. Phospholipid-polymer hybrid nanoparticle-mediated transfollicular delivery of quercetin: Prospective implement for the treatment of androgenic alopecia. Drug Dev. Ind. Pharm. 2019, 45, 1654–1663. [Google Scholar] [CrossRef]
- Fong, P.; Tong, H.H.; Ng, K.H.; Lao, C.K.; Chong, C.I.; Chao, C.M. In silico prediction of prostaglandin D2 synthase inhibitors from herbal constituents for the treatment of hair loss. J. Ethnopharmacol. 2015, 175, 470–480. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, Q.; Zhang, Y.; Zhuang, H.; Wang, E.; Xu, Q.; Ma, L.; Wu, C.; Huan, Z.; Guo, F.; et al. Design of a Multifunctional Biomaterial Inspired by Ancient Chinese Medicine for Hair Regeneration in Burned Skin. ACS Appl. Mater. Interfaces 2020, 12, 12489–12499. [Google Scholar] [CrossRef]
- Kwon, O.S.; Han, J.H.; Yoo, H.G.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine 2007, 14, 551–555. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Up No, S.; Kim, M.H.; Kim, H.S.; Kang, H.; Kim, H.O.; Park, Y.M. Effects of topical application of EGCG on testosterone-induced hair loss in a mouse model. Exp. Dermatol. 2011, 20, 1015–1017. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Park, S.Y.; Song, H.G.; Hwang, E.; Lee, D.G.; Yi, T.H. The Androgenic Alopecia Protective Effects of Forsythiaside-A and the Molecular Regulation in a Mouse Model. Phytother. Res. 2015, 29, 870–876. [Google Scholar] [CrossRef]
- Wang, T.L.; Zhou, C.; Shen, Y.W.; Wang, X.Y.; Ding, X.L.; Tian, S.; Liu, Y.; Peng, G.H.; Xue, S.Q.; Zhou, J.E.; et al. Prevalence of androgenetic alopecia in China: A community-based study in six cities. Br. J. Dermatol. 2010, 162, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Cha, H.J.; Kim, K.; An, I.-S.; Kim, K.-Y.; Ku, J.-E.; Jeong, S.-H.; An, S. Epigallocatechin-3-gallate inhibits paclitaxel-induced apoptosis through the alteration of microRNA expression in human dermal papilla cells. Biomed. Dermatol. 2018, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Guangdong LIYI Technology Co. Ltd. A method of HHORSCs Exosomes Promoting Dermal Papilla Cells to Induce Hair Regeneration. CN202210069596.5, 3 June 2022. [Google Scholar]
- Harbin Medical University. Application of LED-Red Light with 610 nm–650 nm Wavelengths in Alopecia Treatment. CN201711166676.8, 4 April 2023. [Google Scholar]
- Li, R.Y. Equipment for Laser Therapy on Androgenetic Alopecia and Alopecia Treatment Methods. CN202010582452.0, 23 June 2020. [Google Scholar]
- Biorchestra Co. Ltd. Methods and Compositions for Aesthetic and Cosmetic Treatment and Stimulating Hair Growth. US17693457, 14 March 2022. [Google Scholar]
- Biorchestra Co. Ltd. Use of miRNA-485 Inhibitors for Inducing Hair Regrowth. PCT/IB2021/062226, 23 December 2021. [Google Scholar]
- Shenzhen Cell Inspire Pharmaceutical Dev Co. Ltd. Hydrazone Amide Derivative and Application Thereof in the Preparation of Medicaments for Preventing and Treating Alopecia. US17562976, 27 December 2021. [Google Scholar]












| Factors | Molecular of Action | Action Site | Results | Mechanisms | References |
|---|---|---|---|---|---|
| microRNA | miR122 | Hair follicle | Induces hDPC apoptosis | Repression of IGF1R | [21] |
| miR24 | Hair follicle progenitors | miR24 limits the sensitivity of hair follicle progenitors to growth stimuli | Downregulation of PIK3 and CCNE1 | [22] | |
| miR218-5p | HFs | Accelerates the onset of anagen | Downregulates SFRP2 to promote β-catenin expression | [23] | |
| Hedgehog signaling | Lepr | DPCs | Induces new hair growth and hair multiplication | Downstream SCUBE3-TGF-β signaling | [24] |
| mTORC2-Akt signaling | \ | Hair follicle stem cell niche | Allows progenitors to return to the hypoxic niche Resumes the stem cell state | \ | [25] |
| Immune system | Glucocorticoids | Treg cells | Facilitates HFSC activation and hair follicle regeneration | GR and Foxp3 cooperatively induce TGF-β3 to activate Smad2/3 in HFSCs | [26] |
| Corticosterone | DPCs | Prolongs HFSC quiescence Keeps hair follicles in an extended resting phase | Inhibits Gas6 | [27] | |
| Regulatory T cells | HFs | Augments HFSC proliferation and differentiation | Induces high levels of the Notch ligand family member Jagged 1 | [28] | |
| Others | Sirt7 | HFSCs | Facilitates the onset of the hair cycle | Promotes NFATc1 degradation | [29] |
| Photobiomodulation therapy | β-catenin | HFSCs | Drives quiescent HFSC activation Alleviates hair follicle atrophy | Induces ROS and activates the PI3K/AKT/GSK-3β signaling pathways to inhibit β-catenin degradation | [30] |
| Electrical stimulation | \ | HFs | Increases hair follicle number | Improves the secretion of VEGF and KGF | [31] |
| Mechanical stretch | \ | HFSCs | Activates stem cells and promotes hair regeneration | M2 macrophages release growth factor HGF and IGF-1 | [32] |
| External Stimulation | Mechanisms | Effects | Characteristics/Advantages | Limitations | References |
|---|---|---|---|---|---|
| Light (Photostimulation) | Increasing the mitochondrial membrane potential Producing ROS to activate cell proliferation and wound healing | Activates the anagen phase and the proliferation of hair follicles | No thermal/inflammatory tissue damage | High energy consumption Large equipment size | [36,37,38] |
| Ultrasound | Thermal, cavitation, and acoustic streaming | Delivers drugs into dermal papilla cells | Low cost, non-invasive, good penetration | Thermal damage | [39] |
| Electric current | Activating Wnt/β-catenin and MAPK pathways and the secretion of various growth factors | Improves hair follicle density and increases hair shaft length | Low-cost, stable, and small equipment size | Skin damage | [40,41] |
| Stretch | Activating Wnt and BPM-2 pathways Recruiting Macrophages and inducing the secretion of various growth factors | Activates hair follicle stem cells | Small equipment size and high compliance | \ | [32,42] |
| Imaging Technique | Depth | Principle | Features | Drawbacks | Applications | References |
|---|---|---|---|---|---|---|
| Optical coherence tomography | <2 mm | Continuous-wave infrared laser radiation | Qualitative Non-invasive | Low resolution, insufficient for observing cell morphology | Vasculature information enables the assessment of inflammatory skin diseases | [81] |
| Confocal laser scanning microscopy (CLSM) | 200 μm | \ | Qualitative | Limited to molecules with a fluorophore | Observation of cellular structures | [82] |
| Ultrasonography | 10 cm | \ | Qualitative High-resolution | \ | Location of vessels | [83] |
| Mass spectrometry imaging (DESI)-MSI | 50–200 µm | Ionization technique | High-throughput Minimum sample preparation Quick results | Lower spatial resolution and sensitivity | Quantitative information Direct analysis of drug distribution Investigation of drug penetration | [84,85] |
| RSOM | 1.5–5 mm | Light absorption | Qualitative Non-invasive | Longer scan times | Observation of vascular structures | [86] |
| 3D OrbiSIMS | 1–2 µm | Collection of secondary ions | Quantitative High-resolution | Can only analyze standard compounds | Examination of endogenous species | [87] |
| Herbal Bioactive | Plant Origin | Mechanism of Action | Study Model | Dosage | Conclusions | References |
|---|---|---|---|---|---|---|
| Tectoridin | Rhizoma belamcandae | Activating Wnt/β-catenin signaling in human dermal papilla cells | Human follicular DPCs/mouse vibrissae organ | Cells: 3, 10, 20, and 50 µM Mouse vibrissae organ: 50 or 100 µM | Tectoridin promoted hair growth in a dose-dependent manner | [90] |
| Cepharanthine | Stephania cephalantha | Stimulating the growth of human DPCs (hDPCs), significantly increasing the expression of VEGF and the concentration of intracellular Ca2+, as well as increasing the expression of HIF-1α and HIF-2α and HIF-responsive genes in hDPCs | hDPCs | 0.625/1.25/2.5 μg/mL | Cepharanthine promoted the proliferation of hDPCs and increased the expression of VEGF | [91,92] |
| Astragaloside IV | Astragalus membranaceus | Blocking the Fas/Fas L-mediated apoptotic pathway, blocking procaspase-8, and inhibiting caspase-3 and procaspase-9 activities, thus causing the downregulation of Bax and p53, upregulation of Bcl-2 and Bcl-xL, and inhibition of NF-κB and IkB-α phosphorylation, accompanied by a decrease in the levels of three MAPKs: ERK, SAPK/JNK, and p38 | Seven-week-old female C57BL/6 mice (18–20 g) | 1 mM or 100 mM | Astragaloside IV inhibited apoptosis-regression catagen in hair follicles via the Fas/Fas L-mediated cell death pathway, the terminal differentiation of hair keratinocytes, and the levels of regeneration factors and cytokines, leading to hair regeneration. | [93] |
| Acankoreoside J | Acanthopanax koreanum | Increasing nuclear β-catenin levels, upregulating cyclin D1, cyclin E, and CDK2, and downregulating p27kip1 in DPCs, thus promoting DPC proliferation | DPCs male Wistar rats (23 days old) | Rat vibrissa dermal papilla cells were treated with acankoreoside J (AK10) at 0.1, 0.2, 1, and 2 µM for 4 days; rat vibrissa follicles were treated with acankoreoside J (AK10) at 0.1, 1, and 10 µM for 21 days | The proliferation of DPCs and the regeneration of hair fibers in rat vibrissa follicles were increased. Hence, acankoreoside J (AK10) might have therapeutic potential for the promotion of hair regeneration | [94] |
| 3-Deoxysappanchalcone | Caesalpinia sappan L. (Leguminosae) | Stimulating hair regeneration likely by inducing the proliferation of follicular DPCs via the modulation of Wnt/β-catenin and STAT signaling | Human hair follicle DPCs; Seven-week-old female C57BL mice | Two hundred microliters of 3-DSC (3 mM) were applied twice daily for 15 days | The proliferation of HHDPCs and mouse hair regeneration increased in vivo | [95] |
| Cedrol | Platycladus orientalis | \ | Six-week-old C57BL/6 mice (body weight 18–20 g) | Different doses (10, 20, 30) mg/mL of cedrol were applied topically to the test area (100 mL) once a day, for 21 days. | Cedrol promoted hair regeneration in a dose-dependent manner | [96,97,98,99] |
| Baicalin | Scutellaria baicalensis | Activating Wnt/β catenin signaling, enhancing ALP expression and activity in human DPCs, and increasing the expression of IGF-1 and VEGF | Eight-week-old female C57BL/6 mice | Different doses (50 and 100 μM) in 50% ethanol 1(50 μL) were applied topically every day for approximately 5 weeks | Baicalin promoted hair regeneration by inducing anagen from telogen in mice | [100,101] |
| Physcion | Polygonum multiflorum | Inhibiting 5α-reductase | Seven-week-old C57BL/6 mice | 100 μL of Physcion solution (5 mg/mouse/day or 2 mg/mouse/day) for 28 days | Physcion could shorten the time of dorsal skin darkening and hair regeneration, improve hair follicle morphology, and significantly increase hair follicle count | [102] |
| Quercetrin | Hottuynia cordata | Activating the MAPK/CREB signaling pathway | hDPCs/mouse vibrissae organ | Cells: 10 and 100 nM, 1 μM Mouse vibrissae organ: 5 and 10 µM | Increased the expression of growth factors such as bFGF, KGF, PDGF-AA, and VEGF, promoting cell proliferation, and increased the phosphorylation of Akt, Erk, and CREB in cultured hDPCs, stimulating hair growth | [103] |
| Quercetin | Rutin | Reducing HSP70 levels in skin lesions of mouse models of heat-induced alopecia | Human umbilical vein endothelial cells (HUVECs) and human hair follicle DPCs (HHDPCs) Female C3H/HeJ breeders (approximately 6 months old) | Mice were injected subcutaneously with 100 μL of 10 μM quercetin in 10% dimethyl sulfoxide (DMSO) in phosphate-buffered saline (PBS) once daily for 8 days. Mice were monitored for 6 weeks for hair regeneration. | Quercetin induced hair regeneration in preexisting alopecic lesions in C3H/HeJ mice with spontaneous AA | [104,105,106,107] |
| EGCG | Green tea | Downregulating the apoptosis of follicular epithelial cells by upregulating phosphorylated Erk and Akt and increasing the Bcl-2/Bax ratio Androgen metabolism: inhibiting 5a-reductase and repressing the transcription of the androgen receptor (AR) gene | B6CBACF1⁄J female mice (7 weeks old) | Topical application of 100 µL 10% EGCG; T (2 mg/day) was injected intradermally, once daily for 5 days a week, for 12 weeks. The experiment lasted 12 days | EGCG stimulated human hair regeneration through dual proliferative and anti-apoptotic effects on DPCs | [108,109] |
| Forsythiaside-A | Forsythia suspensa | Reducing the expression of TGF-β2, caspase-9, and caspase-3 | HHDPCs, human keratinocytes (HaCaTs) | Test group: injected with DHT (30 mg/EA) and orally administered 100 μL of forsythiaside-A (1%) once a day for 35 days | Forsythiaside-A prevented apoptosis, which was induced by DHT and delayed the entry of catagen in androgenic alopecia | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, Q.; Han, Y.; Cheng, G.; Ma, R.; Yan, Z.; Chen, X.; Yu, G.; Chen, T.; Zhang, S. Innovative Strategies for Hair Regrowth and Skin Visualization. Pharmaceutics 2023, 15, 1201. https://doi.org/10.3390/pharmaceutics15041201
Mai Q, Han Y, Cheng G, Ma R, Yan Z, Chen X, Yu G, Chen T, Zhang S. Innovative Strategies for Hair Regrowth and Skin Visualization. Pharmaceutics. 2023; 15(4):1201. https://doi.org/10.3390/pharmaceutics15041201
Chicago/Turabian StyleMai, Qiuying, Yanhua Han, Guopan Cheng, Rui Ma, Zhao Yan, Xiaojia Chen, Guangtao Yu, Tongkai Chen, and Shu Zhang. 2023. "Innovative Strategies for Hair Regrowth and Skin Visualization" Pharmaceutics 15, no. 4: 1201. https://doi.org/10.3390/pharmaceutics15041201
APA StyleMai, Q., Han, Y., Cheng, G., Ma, R., Yan, Z., Chen, X., Yu, G., Chen, T., & Zhang, S. (2023). Innovative Strategies for Hair Regrowth and Skin Visualization. Pharmaceutics, 15(4), 1201. https://doi.org/10.3390/pharmaceutics15041201