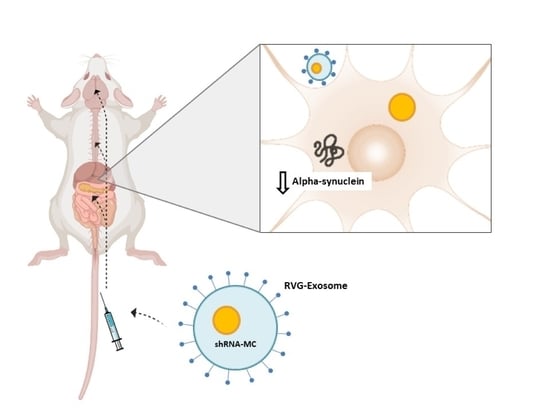
Targeted Extracellular Vesicle Gene Therapy for Modulating Alpha-Synuclein Expression in Gut and Spinal Cord
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Preparation of Mouse Wild-Type Alpha-Synuclein Preformed Fibrils
2.4. Stereotaxic Surgery
2.5. shRNA-Minicircle Generation
2.6. Dendritic Cell Culture and RVG-EV Isolation
2.7. RVG-EV Treatment of Mice
2.8. Western Blot Analysis
2.9. Quantitative PCR
2.10. Immunohistochemistry and Immunofluorescence
2.11. Statistical Analysis
3. Results
3.1. Alpha-Synuclein shRNA-MC RVG-EV Therapy Downregulate Alpha-Synuclein in Spinal Cord and Intestine of Alpha-Synuclein PFF Mouse Model of Parkisnon’s Disease
3.2. Evaluation of Alpha-Synuclein Downregulation by shRNA-MC RVG-EV Administrated after the Development of Alpha-Synuclein Pathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, Q.; Zou, X.; Wang, M.; Wen, Y.; Chen, X.; Weng, X.; Xu, F. Correlation between head tremble and the severity of Parkinson’s disease. CNS Neurosci. Ther. 2021, 28, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Olanow, C.W.; Brundin, P. Parkinson’s Disease and Alpha Synuclein: Is Parkinson’s Disease a Prion-Like Disorder? Mov. Disord. 2013, 28, 31–40. [Google Scholar] [CrossRef]
- Kordower, J.H.; Chu, Y.; Hauser, R.A.; Freeman, T.B.; Olanow, C.W. Lewy body–like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008, 14, 504–506. [Google Scholar] [CrossRef]
- Borghammer, P.; Van Den Berge, N. Brain-First versus Gut-First Parkinson’s Disease: A Hypothesis. J. Park. Dis. 2019, 9, S281–S295. [Google Scholar] [CrossRef] [Green Version]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Steur, E.N.J.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, S.-H.; Kam, T.-I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7. [Google Scholar] [CrossRef]
- Stokholm, M.G.; Danielsen, E.H.; Hamilton-Dutoit, S.J.; Borghammer, P. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann. Neurol. 2016, 79, 940–949. [Google Scholar] [CrossRef]
- Svensson, E.; Horváth-Puhó, E.; Thomsen, R.W.; Djurhuus, J.C.; Pedersen, L.; Borghammer, P.; Sørensen, H.T. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol. 2015, 78, 522–529. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Izco, M.; Blesa, J.; Schleef, M.; Schmeer, M.; Porcari, R.; Al-Shawi, R.; Ellmerich, S.; de Toro, M.; Gardiner, C.; Seow, Y.; et al. Systemic Exosomal Delivery of shRNA Minicircles Prevents Parkinsonian Pathology. Mol. Ther. 2019, 27, 2111–2122. [Google Scholar] [CrossRef]
- Mayrhofer, P.; Blaesen, M.; Schleef, M.; Jechlinger, W. Minicircle-DNA production by site specific recombination and protein-DNA interaction chromatography. J. Gene Med. 2008, 10, 1253–1269. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [Green Version]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release 2014, 199, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, C.; Viskontas, M.; Janowicz, K.; Sani, Y.; Håkansson, M.; Heidari, A.; Huang, W.; Bo, X. The potential of gene therapies for spinal cord injury repair: A systematic review and meta-analysis of pre-clinical studies. Neural Regen. Res. 2023, 18, 299. [Google Scholar] [CrossRef]
- Ito, M.; Natsume, A.; Takeuchi, H.; Shimato, S.; Ohno, M.; Wakabayashi, T.; Yoshida, J. Type I Interferon Inhibits Astrocytic Gliosis and Promotes Functional Recovery after Spinal Cord Injury by Deactivation of the MEK/ERK Pathway. J. Neurotrauma 2009, 26, 41–53. [Google Scholar] [CrossRef]
- Van Der Marel, S. Gene and cell therapy based treatment strategies for inflammatory bowel diseases. World J. Gastrointest. Pathophysiol. 2011, 2, 114–122. [Google Scholar] [CrossRef]
- Verma, P.; Srivastava, A.; Srikanth, C.V.; Bajaj, A. Nanoparticle-mediated gene therapy strategies for mitigating inflammatory bowel disease. Biomater. Sci. 2020, 9, 1481–1502. [Google Scholar] [CrossRef]
- Bhavsar, M.D.; Amiji, M.M. Oral IL-10 gene delivery in a microsphere-based formulation for local transfection and therapeutic efficacy in inflammatory bowel disease. Gene Ther. 2008, 15, 1200–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogy, M.A.; Beinhauer, B.G.; Reinisch, W.; Huang, L.; Pokieser, P. Transfer of Interleukin-4 and Interleukin-10 in Patients with Severe Inflammatory Bowel Disease of the Rectum. Hum. Gene Ther. 2000, 11, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Xing, Z.; Hogaboam, C.M.; Gauldie, J.; Collins, S.M. Interleukin 10 gene transfer prevents experimental colitis in rats. Gut 2000, 46, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katayama, K.; Wada, K.; Nakajima, A.; Mizuguchi, H.; Hayakawa, T.; Nakagawa, S.; Kadowaki, T.; Nagai, R.; Kamisaki, Y.; Blumberg, R.S.; et al. A novel PPARγ gene therapy to control inflammation associated with inflammatory bowel disease in a murine model. Gastroenterology 2003, 124, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.O.; Ciesielski, C.J.; Scheinin, T.; Hodgson, H.J.; Brennan, F.M. The Prevention and Treatment of Murine Colitis Using Gene Therapy with Adenoviral Vectors Encoding IL-10. J. Immunol. 2001, 166, 7625–7633. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, J.; Van Montfrans, C.; Brennan, F.; Van Deventer, S.; Drillenburg, P.; Hodgson, H.; Velde, A.T.; Pena, M.S.R. IL-10 gene therapy prevents TNBS-induced colitis. Gene Ther. 2002, 9, 1715–1721. [Google Scholar] [CrossRef] [Green Version]
- Shao, G.; Greathouse, K.; Huang, Q.; Wang, C.-M.; Sferra, T.J. Gene Transfer to the Gastrointestinal Tract after Peroral Administration of Recombinant Adeno-associated Virus Type 2 Vectors. J. Craniofacial Surg. 2006, 43, 168–179. [Google Scholar] [CrossRef]
- During, M.J.; Xu, R.; Young, D.; Kaplitt, M.G.; Sherwin, R.S.; Leone, P. Peroral gene therapy of lactose intolerance using an adeno-associated virus vector. Nat. Med. 1998, 4, 1131–1135. [Google Scholar] [CrossRef]
- Lau, C.; Soriano, H.E.; Ledley, F.D.; Finegold, M.J.; Wolfe, J.H.; Birkenmeier, E.H.; Henning, S.J. Retroviral Gene Transfer into the Intestinal Epithelium. Hum. Gene Ther. 1995, 6, 1145–1151. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kimura, T.; Haga, K.; Kasahara, N.; Anton, P.; McGowan, I. Effective in vivo and ex vivogene transfer to intestinal mucosa by VSV-G-pseudotyped lentiviral vectors. BMC Gastroenterol. 2010, 10, 44. [Google Scholar] [CrossRef] [Green Version]
- Polyak, S.; Mah, C.; Porvasnik, S.; Herlihy, J.-D.; Campbell-Thompson, M.; Byrne, B.J.; Valentine, J.F. Gene Delivery to Intestinal Epithelial Cells In vitro and In vivo with Recombinant Adeno-Associated Virus Types 1, 2 and 5. Dig. Dis. Sci. 2007, 53, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Nguyen, P.; Jia, F.; Hu, S.; Gong, Y.; de Almeida, P.E.; Wang, L.; Nag, D.; Kay, M.A.; Giaccia, A.J.; et al. Double Knockdown of Prolyl Hydroxylase and Factor-Inhibiting Hypoxia-Inducible Factor with Nonviral Minicircle Gene Therapy Enhances Stem Cell Mobilization and Angiogenesis after Myocardial Infarction. Circulation 2011, 124, S46–S54. [Google Scholar] [CrossRef] [Green Version]
- Meissner, W. When does Parkinson’s disease begin? From prodromal disease to motor signs. Rev. Neurol. 2012, 168, 809–814. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izco, M.; Schleef, M.; Schmeer, M.; Carlos, E.; Verona, G.; Alvarez-Erviti, L. Targeted Extracellular Vesicle Gene Therapy for Modulating Alpha-Synuclein Expression in Gut and Spinal Cord. Pharmaceutics 2023, 15, 1230. https://doi.org/10.3390/pharmaceutics15041230
Izco M, Schleef M, Schmeer M, Carlos E, Verona G, Alvarez-Erviti L. Targeted Extracellular Vesicle Gene Therapy for Modulating Alpha-Synuclein Expression in Gut and Spinal Cord. Pharmaceutics. 2023; 15(4):1230. https://doi.org/10.3390/pharmaceutics15041230
Chicago/Turabian StyleIzco, Maria, Martin Schleef, Marco Schmeer, Estefania Carlos, Guglielmo Verona, and Lydia Alvarez-Erviti. 2023. "Targeted Extracellular Vesicle Gene Therapy for Modulating Alpha-Synuclein Expression in Gut and Spinal Cord" Pharmaceutics 15, no. 4: 1230. https://doi.org/10.3390/pharmaceutics15041230
APA StyleIzco, M., Schleef, M., Schmeer, M., Carlos, E., Verona, G., & Alvarez-Erviti, L. (2023). Targeted Extracellular Vesicle Gene Therapy for Modulating Alpha-Synuclein Expression in Gut and Spinal Cord. Pharmaceutics, 15(4), 1230. https://doi.org/10.3390/pharmaceutics15041230