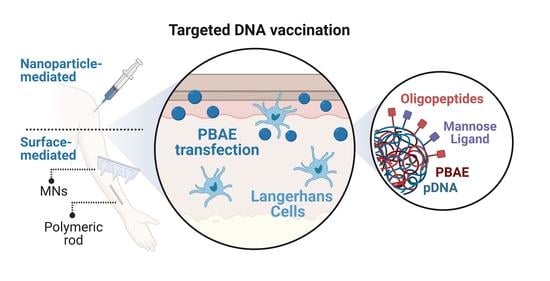
Poly(β-amino ester)s-Based Delivery Systems for Targeted Transdermal Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Synthesis of Oligopeptide-Modified PBAE Polymers
2.4. Synthesis of Mannose-Modified PBAE Polymers
2.5. Formation and Physicochemical Characterization of PBAE-Derived Polyplexes
2.6. Fabrication of Polymeric Rods
2.7. Fabrication of Solid and Dissolvable Microneedles
2.8. Layer-by-Layer Deposition of PBAEs and Characterization on Transcutaneous Delivery Devices
2.9. Ex Vivo Skin Penetration and Film Deposition Studies
2.10. Transfection Efficiency Studies In Vitro
2.11. Statistical Analysis
3. Results and Discussion
3.1. Decoration of Oligopeptide-Modified PBAEs with Mannose Moieties for APC-Targeting
3.2. Characterization of OM- and MM-PBAEs
3.3. Gene Delivery Studies in Professional APCs (Langerhans Cells)
3.4. Gene Delivery Studies in Non-Professional APCs
3.5. Surface-Mediated Gene Delivery Using Multilayer Polyelectrolyte PBAE Films
3.6. PBAE-Based Delivery Systems Can Be Integrated with Transdermal Devices for Dermal Delivery
3.7. Targeted LCs Transfection with OM-/MM-PBAEs Induce Superior Gene Delivery for DNA Vaccination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The Current Landscape of Nucleic Acid Therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The Clinical Progress of MRNA Vaccines and Immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. MRNA Vaccines for Infectious Diseases: Principles, Delivery and Clinical Translation. Nat. Rev. Drug. Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. MRNA Vaccines Manufacturing: Challenges and Bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines—A New Era in Vaccinology. Nature 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Hettinga, J.; Carlisle, R. Vaccination into the Dermal Compartment: Techniques, Challenges, and Prospects. Vaccines 2020, 8, 534. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Transcutaneous Immunization: An Overview of Advantages, Disease Targets, Vaccines, and Delivery Technologies. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 175–201. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Valdivia-Olivares, R.Y.; Rodriguez-Fernandez, M.; Álvarez-Figueroa, M.J.; Kalergis, A.M.; González-Aramundiz, J.V. The Importance of Nanocarrier Design and Composition for an Efficient Nanoparticle-Mediated Transdermal Vaccination. Vaccines 2021, 9, 1420. [Google Scholar] [CrossRef]
- Pielenhofer, J.; Sohl, J.; Windbergs, M.; Langguth, P.; Radsak, M.P. Current Progress in Particle-Based Systems for Transdermal Vaccine Delivery. Front. Immunol. 2020, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.G.; Lynn, D.M.; Langer, R. Semi-Automated Synthesis and Screening of a Large Library of Degradable Cationic Polymers for Gene Delivery. Angew. Chem. Int. Ed. 2003, 42, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J.; Chiu, E.; Leshchiner, E.S.; Shi, J.; Langer, R.; Anderson, D.G. Electrostatic Ligand Coatings of Nanoparticles Enable Ligand-Specific\rGene Delivery to Human Primary Cells. Nano Lett. 2007, 7, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Zugates, G.T.; Tedford, N.C.; Zumbuehl, A.; Jhunjhunwala, S.; Kang, C.S.; Griffith, L.G.; Lauffenburger, D.A.; Langer, R.; Anderson, D.G. Gene Delivery Properties of End-Modified Poly (Beta-Amino Ester)s. Bioconjug. Chem. 2007, 18, 1887–1896. [Google Scholar] [CrossRef]
- Sunshine, J.; Green, J.J.; Mahon, K.P.; Yang, F.; Eltoukhy, A.A.; Nguyen, D.N.; Langer, R.; Anderson, D.G. Small-Molecule End-Groups of Linear Polymer Determine Cell-Type Gene-Delivery Efficacy. Adv. Mater. 2009, 21, 4947–4951. [Google Scholar] [CrossRef]
- Segovia, N.; Dosta, P.; Cascante, A.; Ramos, V.; Borrós, S. Oligopeptide-Terminated Poly (b-Amino Ester) s for Highly Efficient Gene Delivery and Intracellular Localization. Acta Biomater. 2014, 10, 2147–2158. [Google Scholar] [CrossRef]
- Dosta, P.; Segovia, N.; Cascante, A.; Ramos, V.; Borrós, S. Surface Charge Tunability as a Powerful Strategy to Control Electrostatic Interaction for High Efficiency Silencing, Using Tailored Oligopeptide-Modified Poly(Beta-Amino Ester)s (PBAEs). Acta Biomater. 2015, 20, 82–93. [Google Scholar] [CrossRef]
- Stoitzner, P.; Sparber, F.; Tripp, C.H. Langerhans Cells as Targets for Immunotherapy against Skin Cancer. Immunol. Cell. Biol. 2010, 88, 431–437. [Google Scholar] [CrossRef]
- Romani, N.; Flacher, V.; Tripp, C.H.; Sparber, F.; Ebner, S.; Stoitzner, P. Targeting Skin Dendritic Cells to Improve Intradermal Vaccination. Curr. Top. Microbiol. Immunol. 2012, 351, 113–138. [Google Scholar] [CrossRef]
- Valladeau, J.; Ravel, O.; Dezutter-Dambuyant, C.; Moore, K.; Kleijmeer, M.; Liu, Y.; Duvert-Frances, V.; Vincent, C.; Schmitt, D.; Davoust, J.; et al. Langerin, a Novel C-Type Lectin Specific to Langerhans Cells, Is an Endocytic Receptor That Induces the Formation of Birbeck Granules. Immunity 2000, 12, 71–81. [Google Scholar] [CrossRef]
- Irache, J.M.; Salman, H.H.; Gamazo, C.; Espuelas, S. Mannose-Targeted Systems for the Delivery of Therapeutics. Expert. Opin. Drug. Deliv. 2008, 5, 703–724. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Qu, Y.; Dong, Z.; Zhao, J.; Khan, A.R.; Rehman, S.; Zhao, Z. Poly (Β-Amino Esters) Based Potential Drug Delivery and Targeting Polymer; An Overview and Perspectives. Eur. Polym. J. 2020, 141, 110097. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Keskin, D.; Shi, L. Poly (β-Amino Esters): Synthesis, Formulations, and Their Biomedical Applications. Adv. Healthc. Mater. 2019, 8, 1801359. [Google Scholar] [CrossRef] [PubMed]
- Demuth, P.C.; Min, Y.; Huang, B.; Kramer, J.A.; Miller, A.D.; Barouch, D.H.; Hammond, P.T.; Irvine, D.J. Polymer Multilayer Tattooing for Enhanced DNA Vaccination. Nat. Mater. 2013, 12, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Kim, B.S.; Kim, S.R.; Hammond, P.T.; Irvine, D.J. Layer-by-Layer-Assembled Multilayer Films for Transcutaneous Drug and Vaccine Delivery. ACS Nano 2009, 3, 3719–3729. [Google Scholar] [CrossRef]
- Demuth, P.C.; Su, X.; Samuel, R.E.; Hammond, P.T.; Irvine, D.J. Nano-Layered Microneedles for Transcutaneous Delivery of Polymer Nanoparticles and Plasmid DNA. Adv. Mater. 2010, 22, 4851–4856. [Google Scholar] [CrossRef]
- Guillot, A.J.; Cordeiro, A.S.; Donnelly, R.F.; Montesinos, M.C.; Garrigues, T.M.; Melero, A. Microneedle-based Delivery: An Overview of Current Applications and Trends. Pharmaceutics 2020, 12, 569. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent Advances of Microneedles for Biomedical Applications: Drug Delivery and Beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.H.; Prausnitz, M.R. Microneedles for Drug and Vaccine Delivery. Adv. Drug. Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]
- Dosta, P.; Puigmal, N.; Cryer, A.M.; Rodríguez, A.L.; Scott, E.; Weissleder, R.; Miller, M.A.; Artzi, N. Polymeric Microneedles Enable Simultaneous Delivery of Cancer Immunomodulatory Drugs and Detection of Skin Biomarkers. Theranostics 2023, 13, 1–15. [Google Scholar] [CrossRef]
- Du, G.; Hathout, R.M.; Nasr, M.; Nejadnik, M.R.; Tu, J.; Koning, R.I.; Koster, A.J.; Slütter, B.; Kros, A.; Jiskoot, W.; et al. Intradermal Vaccination with Hollow Microneedles: A Comparative Study of Various Protein Antigen and Adjuvant Encapsulated Nanoparticles. J. Control. Release 2017, 266, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Dosta, P.; Ramos, V.; Borrós, S. Stable and Efficient Generation of Poly(β-Amino Ester)s for RNAi Delivery. Mol. Syst. Des. Eng. 2018, 3, 677–689. [Google Scholar] [CrossRef]
- Bechler, S.L.; Lynn, D.M. Characterization of Degradable Polyelectrolyte Multilayers Fabricated Using DNA and a Fluorescently-Labeled Poly(B-Amino Ester): Shedding Light on the Role of the Cationic Polymer in Promoting Surface-Mediated Gene Delivery. Biomacromolecules 2012, 13, 542–552. [Google Scholar] [CrossRef]
- Dosta, P.; Tamargo, I.; Ramos, V.; Kumar, S.; Kang, D.W.; Borrós, S.; Jo, H. Delivery of Anti-MicroRNA-712 to Inflamed Endothelial Cells Using Poly(β-Amino Ester) Nanoparticles Conjugated with VCAM-1 Targeting Peptide. Adv. Healthc. Mater. 2021, 10, e2001894. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; Guerra-rebollo, M.; Lázaro, M.Á.; Castells-sala, C.; Meca-cortés, O.; Ramos-pérez, V.; Cascante, A.; Rubio, N.; Blanco, J.; Borrós, S. MRNA Delivery System for Targeting Antigen-Presenting Cells In Vivo. Adv. Healthc. Mater. 2018, 7, 1800335. [Google Scholar] [CrossRef]
- Fornaguera, C.; Guerra-Rebollo, M.; Lázaro, M.Á.; Cascante, A.; Rubio, N.; Blanco, J.; Borrós, S. In Vivo Retargeting of Poly(Beta Aminoester) (OM-PBAE) Nanoparticles Is Influenced by Protein Corona. Adv. Healthc. Mater. 2019, 8, 1900849. [Google Scholar] [CrossRef]
- Dosta, P.; Demos, C.; Ramos, V.; Kang, D.W.; Kumar, S.; Jo, H.; Borrós, S. Delivery of SiRNA to Endothelial Cells In Vivo Using Lysine/Histidine Oligopeptide-Modified Poly(β-Amino Ester) Nanoparticles. Cardiovasc. Eng. Technol. 2021, 12, 114–125. [Google Scholar] [CrossRef]
- Bauer, J.; Bahmer, F.A.; Wörl, J.; Neuhuber, W.; Schuler, G.; Fartasch, M. A Strikingly Constant Ratio Exists Between Langerhans Cells and Other Epidermal Cells in Human Skin. A Stereologic Study Using the Optical Disector Method and the Confocal Laser Scanning Microscope. J. Investig. Dermatol. 2001, 116, 313–318. [Google Scholar] [CrossRef]
- Sunshine, J.C.; Akanda, M.I.; Li, D.; Kozielski, K.L.; Green, J.J. Effects of Base Polymer Hydrophobicity and End-Group Modification on Polymeric Gene Delivery. Biomacromolecules 2011, 12, 3592–3600. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Zhou, C.; Jiao, Y. Hydrophobic Modifications of Cationic Polymers for Gene Delivery. Prog. Polym. Sci. 2010, 35, 1144–1162. [Google Scholar] [CrossRef]
- Incani, V.; Lavasanifar, A.; Uludağ, H. Lipid and Hydrophobic Modification of Cationic Carriers on Route to Superior Gene Vectors. Soft Matter 2010, 6, 2124–2138. [Google Scholar] [CrossRef]
- Jones, C.H.; Chen, M.; Gollakota, A.; Ravikrishnan, A.; Zhang, G.; Lin, S.; Tan, M.; Cheng, C.; Lin, H.; Pfeifer, B.A. Structure−Function Assessment of Mannosylated Poly(β-Amino Esters) upon Targeted Antigen Presenting Cell Gene Delivery. Biomacromolecules 2015, 16, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.H. In Vivo Function of Langerhans Cells and Dermal DC. Trends Immunol. 2010, 31, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Lindstedt, M.; Borrebaeck, C.A.K. Functional and Transcriptional Profiling of MUTZ-3, a Myeloid Cell Line Acting as a Model for Dendritic Cells. Immunology 2006, 117, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Masterson, A.J.; Sombroek, C.C.; de Gruijl, T.D.; Graus, Y.M.F.; van der Vliet, H.J.J.; Lougheed, S.M.; van den Eertwegh, A.J.M.; Pinedo, H.M.; Scheper, R.J. MUTZ-3, a Human Cell Line Model for the Cytokine-Induced Differentiation of Dendritic Cells from CD34+precursors. Blood 2002, 100, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Riedl, E.; Lowenthal, M.S.; Liotta, L.A.; Briner, D.M.; Crouch, E.C.; Udey, M.C. Identification and Characterization of Endogenous Langerin Ligands in Murine Extracellular Matrix. J. Investig. Dermatol. 2006, 126, 1549–1558. [Google Scholar] [CrossRef]
- Santegoets, S.J.A.M.; van den Eertwegh, A.J.M.; van de Loosdrecht, A.A.; Scheper, R.J.; de Gruijl, T.D. Human Dendritic Cell Line Models for DC Differentiation and Clinical DC Vaccination Studies. J. Leukoc. Biol. 2008, 84, 1364–1373. [Google Scholar] [CrossRef]
- Zhang, L.W.; Bäumer, W.; Monteiro-Riviere, N.A. Cellular Uptake Mechanisms and Toxicity of Quantum Dots in Dendritic Cells. Nanomedicine 2011, 6, 777–791. [Google Scholar] [CrossRef]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ Entry into the Cell: Relevance to Drug Delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef]
- Manzanares, D.; Ceña, V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, Y.; Xin, Y.; Jiang, C.; Yan, B.; Zhai, S. Interactions Between Nanoparticles and Dendritic Cells: From the Perspective of Cancer Immunotherapy. Front. Oncol. 2018, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Uzun, O.; Hu, Y.; Hu, Y.; Han, H.; Watson, N.; Chen, S.; Irvine, D.J.; Stellacci, F. Surface-Structure-Regulated Cell-Membrane Penetration by Monolayer-Protected Nanoparticles. Nat. Mater. 2008, 7, 585–595. [Google Scholar] [CrossRef]
- Keler, T.; Ramakrishna, V.; Fanger, M.W. Mannose Receptor-Targeted Vaccines. Expert. Opin. Biol. Ther. 2004, 4, 1953–1962. [Google Scholar] [CrossRef]
- Silva, J.M.; Vandermeulen, G.; Oliveira, V.G.; Pinto, S.N.; Rodrigues, C.; Salgado, A.; Afonso, C.A.; Viana, A.S.; Jérôme, C.; Silva, L.C.; et al. Development of Functionalized Nanoparticles for Vaccine Delivery to Dendritic Cells: A Mechanistic Approach. Nanomedicine 2014, 9, 2639–2656. [Google Scholar] [CrossRef]
- Liard, C.; Munier, S.; Joulin-Giet, A.; Bonduelle, O.; Hadam, S.; Duffy, D.; Vogt, A.; Verrier, B.; Combadière, B. Intradermal Immunization Triggers Epidermal Langerhans Cell Mobilization Required for CD8 T-Cell Immune Responses. J. Investig. Dermatol. 2012, 132, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Heath, W.R.; Carbone, F.R. The Skin-Resident and Migratory Immune System in Steady State and Memory: Innate Lymphocytes, Dendritic Cells and T Cells. Nat. Immunol. 2013, 14, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Kündig, T.M.; Bachmann, M.F.; DiPaolo, C.; Simard, J.J.; Battegay, M.; Lother, H.; Gessner, A.; Kühlcke, K.; Ohashi, P.S.; Hengartner, H. Fibroblasts as Efficient Antigen-Presenting Cells in Lymphoid Organs. Science 1995, 268, 1343–1347. [Google Scholar] [CrossRef]
- Kim, B.S.; Miyagawa, F.; Cho, Y.-H.; Bennett, C.L.; Clausen, B.E.; Katz, S.I. Keratinocytes Function as Accessory Cells for Presentation of Endogenous Antigen Expressed in the Epidermis. J. Investig. Dermatol. 2009, 129, 2805–2817. [Google Scholar] [CrossRef]
- Jones, C.H.; Chen, M.; Ravikrishnan, A.; Reddinger, R.; Zhang, G.; Hakansson, A.P.; Pfeifer, B.A. Mannosylated Poly (Beta-Amino Esters) for Targeted Antigen Presenting Cell Immune Modulation. Biomaterials 2014, 37, 333–344. [Google Scholar] [CrossRef]
- Sheikh, H.; Yarwood, H.; Ashworth, A.; Isacke, C.M. Endo180, an Endocytic Recycling Glycoprotein Related to the Macrophage Mannose Receptor Is Expressed on Fibroblasts, Endothelial Cells and Macrophages and Functions as a Lectin Receptor. J. Cell. Sci. 2000, 113, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Jewell, C.M.; Zhang, J.; Fredin, N.J.; Lynn, D.M. Multilayered Polyelectrolyte Films Promote the Direct and Localized Delivery of DNA to Cells. J. Control. Release 2005, 106, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Jeong, S.S.; Roh, D.H.; Kim, D.Y.; Choi, H.K.; Lee, E.H. A Practical Guide to the Development of Microneedle Systems—In Clinical Trials or on the Market. Int. J. Pharm. 2020, 573, 118778. [Google Scholar] [CrossRef] [PubMed]







| Single OM-/MM-Polyplexes | |||
|---|---|---|---|
| Nomenclature | Oligopeptide 1 | ||
| R | Cys + 3Arg | ||
| K | Cys + 3Lys | ||
| R-Man | Cys + 3Arg-man | ||
| K-Man | Cys + 3Lys-man | ||
| Multiple OM-/MM-polyplexes | |||
| Nomenclature | Oligopeptide 1 | Oligopeptide 2 | Ratio |
| R/D | Cys + 3Arg | Cys + 3Asp | 70:30 |
| K/D | Cys + 3Lys | Cys + 3Asp | 70:30 |
| R/K | Cys + 3Arg | Cys + 3Lys | 50:50 |
| K/H | Cys + 3Lys | Cys + 3His | 50:50 |
| R/H | Cys + 3Arg | Cys + 3His | 50:50 |
| R/D-Man | Cys + 3Arg-man | Cys + 3Asp | 70:30 |
| K/D-Man | Cys + 3Lys-man | Cys + 3Asp | 70:30 |
| R/K-Man | Cys + 3Arg-man | Cys + 3Lys-man | 50:50 |
| R/H-Man | Cys + 3Arg-man | Cys + 3His | 50:50 |
| K/H-Man | Cys + 3Lys-man | Cys + 3His | 50:50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puigmal, N.; Ramos, V.; Artzi, N.; Borrós, S. Poly(β-amino ester)s-Based Delivery Systems for Targeted Transdermal Vaccination. Pharmaceutics 2023, 15, 1262. https://doi.org/10.3390/pharmaceutics15041262
Puigmal N, Ramos V, Artzi N, Borrós S. Poly(β-amino ester)s-Based Delivery Systems for Targeted Transdermal Vaccination. Pharmaceutics. 2023; 15(4):1262. https://doi.org/10.3390/pharmaceutics15041262
Chicago/Turabian StylePuigmal, Núria, Víctor Ramos, Natalie Artzi, and Salvador Borrós. 2023. "Poly(β-amino ester)s-Based Delivery Systems for Targeted Transdermal Vaccination" Pharmaceutics 15, no. 4: 1262. https://doi.org/10.3390/pharmaceutics15041262
APA StylePuigmal, N., Ramos, V., Artzi, N., & Borrós, S. (2023). Poly(β-amino ester)s-Based Delivery Systems for Targeted Transdermal Vaccination. Pharmaceutics, 15(4), 1262. https://doi.org/10.3390/pharmaceutics15041262