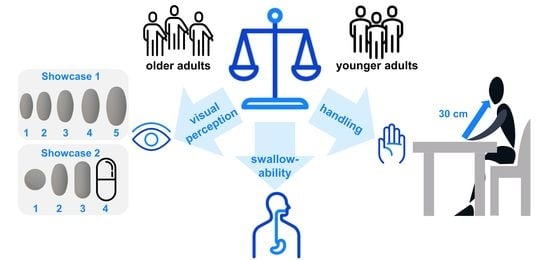
Influence of Solid Oral Dosage Form Characteristics on Swallowability, Visual Perception, and Handling in Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
| Shape | Weight [mg] | Diameter [mm] | Cap Radius [mm] | Thickness [mm] | Min. Cross Sectional Area [mm2] | Deglutition (Group) | Visual Perception | Handling |
|---|---|---|---|---|---|---|---|---|
| Round | 125 | 7.40 | 9.13 | 2.97 | 18.21 | - | x | x |
| Round | 250 | 9.30 | 11.60 | 3.82 | 29.63 | x (1) | x | x |
| Round | 500 | 11.70 | 14.71 | 4.82 | 47.13 | x (2) | x | x |
| Round | 750 | 13.35 | 16.86 | 5.47 | 60.94 | x (2) | x | x |
| Shape | Weight [mg] | Length [mm] | Width [mm] | Thickness [mm] | Min. Cross Sectional Area [mm2] | Deglutition (Group) | Visual Perception | Handling |
| Oval | 125 | 9.50 | 4.95 | 3.30 | 13.89 | - | x | x |
| Oval | 250 | 12.00 | 6.25 | 4.24 | 22.17 | x (2) | x | x |
| Oval | 500 | 15.10 | 7.87 | 5.31 | 35.15 | x (1) | x | x |
| Oval | 750 | 17.30 | 9.02 | 6.12 | 46.12 | x (1) | x | x |
| Oval | 1000 | 19.00 | 9.90 | 6.66 | 55.64 | x (2) | x | x |
| Oval | 1250 | 20.48 | 10.67 | 7.24 | 64.59 | - | x | - |
| Oblong | 125 | 10.30 | 4.47 | 3.03 | 11.19 | - | x | x |
| Oblong | 250 | 12.75 | 5.53 | 3.81 | 17.11 | - | x | x |
| Oblong | 500 | 16.10 | 6.98 | 4.81 | 27.20 | - | x | x |
| Oblong | 750 | 18.50 | 8.02 | 5.48 | 35.96 | - | x | x |
| Oblong | 1000 | 20.50 | 8.89 | 6.07 | 44.24 | x (1) | x | x |
| Oblong | 1250 | 22.20 | 9.63 | 6.61 | 51.84 | - | x | - |
| Capsule Size | Anticipated Weight [mg] | Length [mm] | Diameter [mm] | Min. Cross Sectional Area [mm2] |
|---|---|---|---|---|
| 4 | 125 | 14.3 | 5.05 | 20.03 |
| 3 | 150 | 15.9 | 5.57 | 24.37 |
| 2 | 200 | 18.0 | 6.07 | 28.94 |
| 1 | 250 | 19.4 | 6.63 | 34.52 |
| 0 | 350 | 21.7 | 7.34 | 42.31 |
| 00 | 500 | 23.3 | 8.18 | 52.55 |
2.2. Handling Assessment
2.3. Visual Perception Assessment

2.4. Study Population and Setting
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Handling
3.2.1. Rating
3.2.2. Reasons for Handling Limitations
3.2.3. Characteristics Describing Handling
3.3. Visual Perception
3.3.1. Shape-Showcases
3.3.2. Weight-Showcases
3.3.3. Influence of Showcase Style on Visual Perception
3.3.4. Influence of Sex on Visual Perception
3.4. Visual Perception in Comparison to Actual Deglutition
4. Discussion
| Studied Population | Study Type | Tested Dosage Forms | Acceptable SODF Size 1 | Reference |
|---|---|---|---|---|
| Older adults, mean age of 86 years, n = 938 tablet evaluations | ClinSearch Acceptability Test® | Retrospective analysis of patients’ regular medications, tablets ranged from 4.7–21.5 mm | Older adults w/o swallowing difficulties: tablets in general, older adults with swallowing disorders: only tablets < 6.5 mm | [39] |
| Older adults, mean age of 74 years, n = 156 | Anticipated swallowability according to visual perception | Tablets: round and elongated shapes of 5–13 mm (d or l); hard gelatin capsules: size 4 to 00 | Older adults with swallowing difficulties (11% of study population): tablet sizes < 11–13 mm (d or l) and capsules of size 0 or smaller | [6] |
| Adult patients, mean age of 61.8 ± 15.6 years, n = 1051 | Patients reported swallowing difficulties among their own regular medications | Retrospective analysis of patients’ regular medications | Round tablets ≤ 8.1 mm (d) and ≤ 3.5 mm (h); oval tablets ≤ 13.2 mm (l), ≤6.6 mm (w), and ≤4.6 mm (h); oblong tablets ≤ 13.3 mm (l), ≤6.2 mm (w), and ≤4.9 mm (h) hard capsules: <6.4 mm (d), <17.5 mm (l); soft capsules: <8.0 mm (d), <18.3 mm (l) | [10] |
| Older adults: mean age of 79 years, n = 18; informal carers: mean age of 61 years, n = 7; health/social care professionals: n = 27 | Semi-structured interviews | Tablets: round: 6–10 mm (d); oval: 12–16.5 mm (l), 7–8.9 mm (w); oblong: 18 mm (l), 7 mm (w) | Tablets > 10 mm (d) in case of a round shape should be provided in an elongated shape; good swallowability if tablets are not too thick | [21] |
| Adult patients, n = 278: 23–64 years, n = 53: ≥65 years | Anticipated swallowability according to visual perception | Tablets: flat or arched round: 8.1–15.1 mm (d), 3.1–5.5 mm (h); oblong: 12.2–22.2 mm (l), 6.3–8.9 mm (w), 4.4–7.2 mm (h); oval: 11.2–20.3 mm (l), 7.6–9.8 mm (w), 3.6–7.0 mm (h) | Small tablets: preference for strongly arched round shape; medium tablets: preference for oval shape; large tablets: preference for oblong, oblong curved, and oval shape | [15] |
| Older adults, mean age 81.3 ± 7.3 years, n = 52 | Anticipated swallowability according to visual perception | Tablets: round: 7.40–13.35 mm (d); oval: 9.50–20.48 mm (l); oblong: 10.30–22.2 mm (l); capsules: size 4 to 00; mini tablets: 750–1000 mg | Swallowable: round tablets ≤ 9.30 mm (d); oval tablets ≤ 12.00 mm (l); oblong tablets ≤ 12.75 mm (l); capsules ≤ size 3 (≤15.9 mm (l)); mini tablets ≤ 1000 mg | Present study |
| Younger adults, mean age 23.1 ± 3.8 years, n = 52 | Swallowable: round tablets ≤ 11.70 mm (d), oval tablets ≤ 17.3 mm (l), oblong tablets ≤ 20.5 mm (l) capsules ≤ size 0 (≤21.7 mm (l)) mini tablets ≤ 1000 mg |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hummler, H.; Stillhart, C.; Meilicke, L.; Grimm, M.; Krause, E.; Mannaa, M.; Gollasch, M.; Weitschies, W.; Page, S. Impact of tablet size and shape on the swallowability in older adults. Pharmaceutics 2023, 15, 1042. [Google Scholar] [CrossRef]
- Bourdenet, G.; Giraud, S.; Artur, M.; Dutertre, S.; Dufour, M.; Lefèbvre-Caussin, M.; Proux, A.; Philippe, S.; Capet, C.; Fontaine-Adam, M.; et al. Impact of recommendations on crushing medications in geriatrics: From prescription to administration. Fundam. Clin. Pharmacol. 2015, 29, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Haw, C.; Stubbs, J. Administration of medicines in food and drink: A study of older inpatients with severe mental illness. Int. Psychogeriatr. 2010, 22, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Strachan, I.; Greener, M. Medication-related swallowing difficulties may be more common than we realise. Pharm. Pract. 2005, 15, 411–414. [Google Scholar]
- Liu, F.; Ranmal, S.; Batchelor, H.K.; Orlu-Gul, M.; Ernest, T.B.; Thomas, I.W.; Flanagan, T.; Tuleu, C. Patient-centered pharmaceutical design to improve acceptability of medicines: Similarities and differences in paediatric and geriatric populations. Drugs 2014, 74, 1871–1889. [Google Scholar] [CrossRef]
- Liu, F.; Ghaffur, A.; Bains, J.; Hamdy, S. Acceptability of oral solid medicines in older adults with and without dysphagia: A nested pilot validation questionnaire based observational study. Int. J. Pharm. 2016, 512, 374–381. [Google Scholar] [CrossRef]
- EMA. Reflection Paper on the Pharmaceutical Development of Medicines for Use in the Older Population. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-pharmaceutical-development-medicines-use-older-population-first-version_en.pdf (accessed on 2 October 2021).
- Stegemann, S.; Sheehan, L.; Rossi, A.; Barrett, A.; Paudel, A.; Crean, A.; Ruiz, F.; Bresciani, M.; Liu, F.; Shariff, Z.; et al. Rational and practical considerations to guide a target product profile for patient-centric drug product development with measurable patient outcomes—A proposed roadmap. Eur. J. Pharm. Biopharm. 2022, 177, 81–88. [Google Scholar] [CrossRef]
- FDA. Size, Shape, and Other Physical Attributes of Generic Tablets and Capsules. Available online: https://www.fda.gov/files/drugs/published/Size--Shape--and-Other-Physical-Attributes-of-Generic-Tablets-and-Capsules.pdf?next=/answers/six-tips-to-avoid-getting-pill-stuck-in-your-throat/avoid-pill-getting-stuck-in-throat/ (accessed on 20 September 2022).
- Schiele, J.T.; Quinzler, R.; Klimm, H.-D.; Pruszydlo, M.G.; Haefeli, W.E. Difficulties swallowing solid oral dosage forms in a general practice population: Prevalence, causes, and relationship to dosage forms. Eur. J. Clin. Pharmacol. 2013, 69, 937–948. [Google Scholar] [CrossRef]
- Lau, E.T.L.; Steadman, K.J.; Mak, M.; Cichero, J.A.Y.; Nissen, L.M. Prevalence of swallowing difficulties and medication modification in customers of community pharmacists. J. Pharm. Pract. Res. 2015, 45, 18–23. [Google Scholar] [CrossRef]
- Souza, L.F.; Nascimento, W.V.; Alves, L.M.T.; Silva, A.C.V.; Cassiani, R.A.; Alves, D.C.; Dantas, R.O. Medication swallowing difficulties in people without dysphagia. Rev. CEFAC 2019, 21, e0119. [Google Scholar] [CrossRef]
- Franko, D.L.; Shapiro, J.; Gagne, A. Phagophobia: A form of psychogenic dysphagia a new entity. Ann. Otol. Rhinol. Laryngol. 1997, 106, 286–290. [Google Scholar] [CrossRef]
- McCloskey, A.P.; Penson, P.E.; Tse, Y.; Abdelhafiz, M.A.; Ahmed, S.N.; Lim, E.J. Identifying and addressing pill aversion in adults without physiological-related dysphagia: A narrative review. Br. J. Clin. Pharmacol. 2022, 88, 5128–5148. [Google Scholar] [CrossRef]
- Overgaard, A.B.A.; Møller-Sonnergaard, J.; Christrup, L.L.; Højsted, J.; Hansen, R. Patients’ evaluation of shape, size and colour of solid dosage forms. Pharm. World Sci. 2001, 23, 185–188. [Google Scholar] [CrossRef]
- Van Santen, E.; Barends, D.M.; Frijlink, H.W. Breaking of scored tablets: A review. Eur. J. Pharm. Biopharm. 2002, 53, 139–145. [Google Scholar] [CrossRef]
- Yetzer, E.; Blake, K.; Goetsch, N.; Shook, M.; Paul, M.S. SAFE medication management for patients with physical impairments of stroke, part one. Rehabil. Nurs. 2015, 40, 260–266. [Google Scholar] [CrossRef]
- Vasylenko, O.; Gorecka, M.M.; Rodríguez-Aranda, C. Manual dexterity in young and healthy older adults. 1. Age- and gender-related differences in unimanual and bimanual performance. Dev. Psychobiol. 2018, 60, 407–427. [Google Scholar] [CrossRef]
- Schenk, A.; Eckardt-Felmberg, R.; Steinhagen-Thiessen, E.; Stegemann, S. Patient behaviour in medication management: Findings from a patient usability study that may impact clinical outcomes. Br. J. Clin. Pharmacol. 2020, 86, 1958–1968. [Google Scholar] [CrossRef]
- Wilson, M.-M.G.; Kaiser, F.E.; Morley, J.E. Tablet-breaking ability of older persons with type 2 diabetes mellitus. Diabetes Educ. 2001, 27, 530–540. [Google Scholar] [CrossRef]
- Shariff, Z.; Kirby, D.; Missaghi, S.; Rajabi-Siahboomi, A.; Maidment, I. Patient-centric medicine design: Key characteristics of oral solid dosage forms that improve adherence and acceptance in older people. Pharmaceutics 2020, 12, 905. [Google Scholar] [CrossRef]
- Yoder, S.; Rajabi, J.; Miller, C.; Oza, K. Physical appearance preferences for oral solid dosage formulations. AAPS Poster 2014, 2018, 1–4. [Google Scholar]
- Gnjidic, D.; Hilmer, S.N.; Blyth, F.M.; Naganathan, V.; Cumming, R.G.; Handelsman, D.J.; McLachlan, A.J.; Abernethy, D.R.; Banks, E.; Le Couteur, D.G. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin. Pharmacol. Ther. 2012, 91, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, C.J.; Smit, E.; Lee, D.S.H.; Alramadhan, F.; Odden, M.C. Polypharmacy among adults aged 65 years and older in the United States: 1988–2010. J. Gerontol. Ser. A 2015, 70, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Schöttker, B.; Saum, K.-U.; Muhlack, D.C.; Hoppe, L.K.; Holleczek, B.; Brenner, H. Polypharmacy and mortality: New insights from a large cohort of older adults by detection of effect modification by multi-morbidity and comprehensive correction of confounding by indication. Eur. J. Clin. Pharmacol. 2017, 73, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef]
- Daniels, S.K.; Brailey, K.; Priestly, D.H.; Herrington, L.R.; Weisberg, L.A.; Foundas, A.L. Aspiration in patients with acute stroke. Arch. Phys. Med. Rehabil. 1998, 79, 14–19. [Google Scholar] [CrossRef]
- Daniels, S.K.; Ballo, L.A.; Mahoney, M.-C.; Foundas, A.L. Clinical predictors of dysphagia and aspiration risk: Outcome measures in acute stroke patients. Arch. Phys. Med. Rehabil. 2000, 81, 1030–1033. [Google Scholar] [CrossRef]
- Shulman, K.I.; Shedletsky, R.; Silver, I.L. The challenge of time: Clock-drawing and cognitive function in the elderly. Int. J. Geriatr. Psychiatry 1986, 1, 135–140. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. False discovery rate–adjusted multiple confidence intervals for selected parameters. J. Am. Stat. Assoc. 2005, 100, 71–81. [Google Scholar] [CrossRef]
- Ruiz, F.; Vallet, T.; Wojcicki, A.D.; Belissa, É.; Fontan, J.-E.; De Pontual, L.; Nathanson, S.; Chevallier, A.; Laribe-Caget, S.; Boudy, V. Dosage form suitability in vulnerable populations: A focus on paracetamol acceptability from infants to centenarians. PLoS ONE 2019, 14, e0221261. [Google Scholar] [CrossRef]
- Walsh, J.; Ranmal, S.R.; Ernest, T.B.; Liu, F. Patient acceptability, safety and access: A balancing act for selecting age-appropriate oral dosage forms for paediatric and geriatric populations. Int. J. Pharm. 2018, 536, 547–562. [Google Scholar] [CrossRef]
- Lorenzini, G.C.; Bell, A.; Olsson, A. ‘You need to be healthy to be sick’: Exploring older people’s experiences with medication packaging at home. Age Ageing 2022, 51, afac050. [Google Scholar] [CrossRef]
- Isaac, L.M.; Tamblyn, R.M.; McGill-Calgary Drug Research Team. Compliance and cognitive function: A methodological approach to measuring unintentional errors in medication compliance in the elderly. Gerontologist 1993, 33, 772–781. [Google Scholar] [CrossRef]
- Vallet, T.; Belissa, E.; Laribe-Caget, S.; Chevallier, A.; Chedhomme, F.-X.; Leglise, P.; Piccoli, M.; Michelon, H.; Bloch, V.; Meaume, S.; et al. A decision support tool facilitating medicine design for optimal acceptability in the older population. Pharm. Res. 2018, 35, 136. [Google Scholar] [CrossRef]
- Vallet, T.; Michelon, H.; Orlu, M.; Jani, Y.; Leglise, P.; Laribe-Caget, S.; Piccoli, M.; Le Fur, A.; Liu, F.; Ruiz, F.; et al. Acceptability in the older population: The importance of an appropriate tablet size. Pharmaceutics 2020, 12, 746. [Google Scholar] [CrossRef]
- Ranmal, S.R.; O’brien, F.; Lopez, F.; Ruiz, F.; Orlu, M.; Tuleu, C.; Walsh, J.; Liu, F. Methodologies for assessing the acceptability of oral formulations among children and older adults: A systematic review. Drug Discov. Today 2018, 23, 830–847. [Google Scholar] [CrossRef]
- Schiele, J.T.; Schneider, H.; Quinzler, R.; Reich, G.; Haefeli, W.E. Two techniques to make swallowing pills easier. Ann. Fam. Med. 2014, 12, 550–552. [Google Scholar] [CrossRef]
- Sam, T.; Ernest, T.B.; Walsh, J.; Williams, J.L.; (EuPFI), on behalf of the E.P.F.I. A benefit/risk approach towards selecting appropriate pharmaceutical dosage forms—An application for paediatric dosage form selection. Int. J. Pharm. 2012, 435, 115–123. [Google Scholar] [CrossRef]










| Showcase | Type of Showcase | Included Dosage Forms |
|---|---|---|
| 1 | shape | Oval shaped tablets— 125 mg, 250 mg, 500 mg, 750 mg, 1000 mg, 1250 mg |
| 2 | shape | Round, biconvex shaped tablets— 125 mg, 250 mg, 500 mg, 750 mg |
| 3 | shape | Oblong shaped tablets— 125 mg, 250 mg, 500 mg, 750 mg, 1000 mg, 1250 mg |
| 4 | weight | Dosage forms of 250 mg weight— oval, round, and oblong shaped tablets as well as one capsule (size 1) |
| 5 | weight | Dosage forms of 500 mg weight— oval, round, and oblong shaped tablets as well as one capsule (size 00) |
| 6 | weight | Dosage forms of 750 mg weight— oval, round, and oblong shaped tablets as well as mini tablets |
| 7 | weight | Dosage forms of 1000 mg weight— oval and oblong shaped tablets as well as mini tablets |
| 8 | shape | Capsules— sizes 4, 3, 2, 1, 0, 00 |
| Tablet | Older Participants | Younger Participants | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean [s] | StdDev [s] | Min [s] | Max [s] | Mean [s] | StdDev [s] | Min [s] | Max [s] | |
| 125 mg round | 3.0 | 1.0 | 2 | 6 | 1.1 | 0.3 | 1 | 3 |
| 250 mg round | 2.9 | 0.7 | 2 | 5 | 1.0 | 0.0 | 1 | 1 |
| 500 mg round | 2.7 | 0.6 | 2 | 4 | 1.0 | 0.0 | 1 | 1 |
| 750 mg round | 3.1 | 1.1 | 2 | 8 | 1.0 | 0.0 | 1 | 1 |
| 125 mg oval | 3.2 | 1.5 | 2 | 12 | 1.0 | 0.2 | 1 | 2 |
| 250 mg oval | 3.2 | 1.6 | 2 | 13 | 1.0 | 0.0 | 1 | 1 |
| 500 mg oval | 3.2 | 1.6 | 2 | 10 | 1.0 | 0.0 | 1 | 1 |
| 750 mg oval | 3.1 | 1.2 | 2 | 8 | 1.0 | 0.0 | 1 | 1 |
| 1000 mg oval | 3.0 | 0.9 | 2 | 5 | 1.0 | 0.0 | 1 | 1 |
| 125 mg oblong | 3.3 | 2.0 | 2 | 16 | 1.1 | 0.2 | 1 | 2 |
| 250 mg oblong | 3.3 | 1.9 | 2 | 14 | 1.0 | 0.1 | 1 | 2 |
| 500 mg oblong | 3.2 | 1.6 | 2 | 13 | 1.0 | 0.0 | 1 | 1 |
| 750 mg oblong | 3.2 | 1.8 | 2 | 14 | 1.0 | 0.0 | 1 | 1 |
| 1000 mg oblong | 2.9 | 0.8 | 2 | 5 | 1.0 | 0.1 | 1 | 2 |
| Older Participants | Younger Participants | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tablet | Number of Attempts [%] | Not to Be Handled [%] | Number of Attempts [%] | Not to Be Handled [%] | ||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |||
| 125 mg round | 88.5 | 3.8 | 7.7 | 0.0 | 0.0 | 0.0 | 96.2 | 1.9 | 1.9 | 0.0 | 0.0 | 0.0 |
| 250 mg round | 92.3 | 5.8 | 1.9 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 500 mg round | 92.3 | 7.7 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 750 mg round | 86.5 | 7.7 | 1.9 | 1.9 | 1.9 | 0.0 | 98.1 | 1.9 | 0.0 | 0.0 | 0.0 | 0.0 |
| 125 mg oval | 75.0 | 23.1 | 0.0 | 0.0 | 0.0 | 1.9 | 96.2 | 1.9 | 1.9 | 0.0 | 0.0 | 0.0 |
| 250 mg oval | 78.8 | 19.2 | 0.0 | 0.0 | 0.0 | 1.9 | 98.1 | 1.9 | 0.0 | 0.0 | 0.0 | 0.0 |
| 500 mg oval | 88.5 | 7.7 | 0.0 | 0.0 | 1.9 | 1.9 | 98.1 | 1.9 | 0.0 | 0.0 | 0.0 | 0.0 |
| 750 mg oval | 82.7 | 15.4 | 1.9 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1000 mg oval | 84.6 | 11.5 | 1.9 | 1.9 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 125 mg oblong | 82.7 | 9.6 | 1.9 | 3.8 | 0.0 | 1.9 | 96.2 | 3.8 | 0.0 | 0.0 | 0.0 | 0.0 |
| 250 mg oblong | 80.8 | 11.5 | 3.8 | 1.9 | 0.0 | 1.9 | 98.1 | 1.9 | 0.0 | 0.0 | 0.0 | 0.0 |
| 500 mg oblong | 80.8 | 15.4 | 1.9 | 0.0 | 0.0 | 1.9 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 750 mg oblong | 86.5 | 7.7 | 1.9 | 0.0 | 1.9 | 1.9 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1000 mg oblong | 90.4 | 3.8 | 5.8 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hummler, H.; Page, S.; Stillhart, C.; Meilicke, L.; Grimm, M.; Mannaa, M.; Gollasch, M.; Weitschies, W. Influence of Solid Oral Dosage Form Characteristics on Swallowability, Visual Perception, and Handling in Older Adults. Pharmaceutics 2023, 15, 1315. https://doi.org/10.3390/pharmaceutics15041315
Hummler H, Page S, Stillhart C, Meilicke L, Grimm M, Mannaa M, Gollasch M, Weitschies W. Influence of Solid Oral Dosage Form Characteristics on Swallowability, Visual Perception, and Handling in Older Adults. Pharmaceutics. 2023; 15(4):1315. https://doi.org/10.3390/pharmaceutics15041315
Chicago/Turabian StyleHummler, Henriette, Susanne Page, Cordula Stillhart, Lisa Meilicke, Michael Grimm, Marwan Mannaa, Maik Gollasch, and Werner Weitschies. 2023. "Influence of Solid Oral Dosage Form Characteristics on Swallowability, Visual Perception, and Handling in Older Adults" Pharmaceutics 15, no. 4: 1315. https://doi.org/10.3390/pharmaceutics15041315
APA StyleHummler, H., Page, S., Stillhart, C., Meilicke, L., Grimm, M., Mannaa, M., Gollasch, M., & Weitschies, W. (2023). Influence of Solid Oral Dosage Form Characteristics on Swallowability, Visual Perception, and Handling in Older Adults. Pharmaceutics, 15(4), 1315. https://doi.org/10.3390/pharmaceutics15041315