Chitosan-Based Particulate Carriers: Structure, Production and Corresponding Controlled Release
Abstract
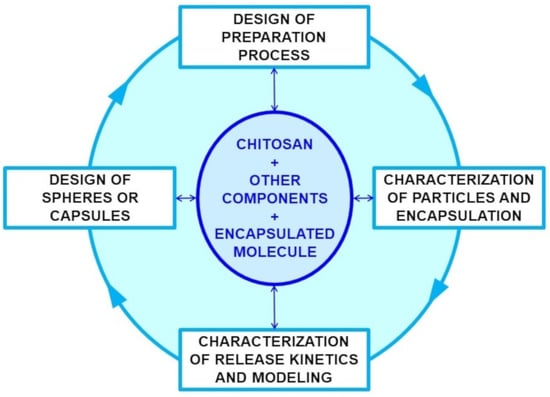
:1. Introduction
1.1. Emerging Research and Industrial Interest for Chitosan
1.2. A Multiple-Application Biopolymer
1.3. Terminology of Particulate Carriers



| Terminology | Concise Definition |
|---|---|
| Nanoparticle | Particle of any shape with at least one characteristic dimension between 10−9 and 10−7 m |
| Nanocapsule | Hollow nanoparticle consisting of a solid shell encircling a core-forming area |
| Nanosphere | Spherical-shaped nanoparticle without membrane or any distinct outer layer |
| Microparticle | Particle with at least one dimension between 10−7 and 10−4 m |
| Microcapsule | Hollow microparticle composed of a solid shell surrounding a core-forming space |
| Microsphere | Microparticle of spherical shape without membrane or any distinct outer layer |
2. Preparation Methods for Chitosan-Based Network and Particulate Structure

| Method | Description | Comments | Particle Dimension | Geometry of Particle | Particle Concentration (w/v) | Key Parameters | References | |
|---|---|---|---|---|---|---|---|---|
| Merit (s) | Demerit (s) | |||||||
| Droplet extrusion method | Droplets of drug-loaded polymeric solution are formed by extrusion through a nozzle into a bath of an aqueous solution of polyvalent cations. | The process is convenient, cost-effective and devoid of high temperatures and use of solvent. Also usable for living-cell encapsulation. | Limited size control/size reduction. Difficulties in large-scale production. Teardrop-shaped particles. | 90 µm–7 mm |  | - | Polymer concentration; viscosity of polymer solution; flow rate; geometry of extrusion device; type and concentration of non-solvent bath. | Microcapsule [67]; microsphere [68]. |
| Crosslinking gelation | Electrostatic interaction between polyelectrolytes and polyvalent ions is often used as the driving force to form micro-/nanoparticles. The positively charged natural polymer CS has been broadly investigated to form composites with negative electrolytes by ionic crosslinking (ionotropic gelation). Alternatively, covalent crosslinking has been used. | Mild processing conditions. Simple equipment. Ionotropic gelation: low toxicity, limited risk of altering the encapsulated drug. | Poor stability in non-acidic conditions. Difficulty in encapsulating high-molecular-weight drugs. Toxicity of certain covalent crosslinkers (aldehydes, for instance). | 10 nm–3 mm |  | 0.02–1.2% | Polymer concentration; crosslinking agent concentration; mixing rate and time; temperature; pH. | Microsphere [45,46,65,69,70,71,72,73,74,75,76,77,78,79,80,81,82]; microcapsule [83,84]. |
| Polyelectrolyte complexation (PEC) | Complexation of CS with synthetic anionic polyelectrolytes or natural anionic biopolymers via electrostatic interaction. | Able to encapsulate macromolecules such as polypeptides and polynucleotides, as well as hydrophobic drugs. | Toxicity of certain covalent crosslinkers (aldehydes, for instance). | 50–450 nm |  | 0.1–2% | Polyelectrolyte concentration; pH; mixing rate and time; temperature; solvent type. | Microsphere [76,84,85]; microcapsule [86]. |
| Complex coacervation/precipitation | CS acetic solution was mixed up with a DNA/protein dissolved salty (sodium sulfate) solution to form micro-/nanospheres. | Narrow particle size distribution; high encapsulation efficiency; relatively low cost of processing. | Safety issue of toxic crosslinkers; poor product formation due to poor solubility of active agent (e.g., plant protein). | 50–1600 nm |  | 0.25–0.7% | Nature and concentration of polyelectrolytes; pH; temperature; solvent and co-solvent. | Microsphere [76,87,88]. |
| Emulsion–coacervation (Emulsion–alkali precipitation) method | Drug/oil mixture is dispersed in CS acidic solution under stirring, followed by ultra-sonication/homogenization to obtain homogeneous emulsion. Microcapsules were obtained by dropping alkaline solution into aforesaid emulsion. | Devoid of crosslinker. | Suitable for lipophilic drug encapsulation. | 10–12 µm |  | 0.05–0.19% | Type and concentration of the polymer, surfactant and alkaline solution; emulsion stirring rate; aging time. | Microcapsule [52,89,90]; double-walled microspheres [78]. |
| Emulsion crosslinking | CS aqueous solution is dispersed into oily phase in the presence of suitable surfactants as emulsion stabilizers. Thermal crosslinking produces microspheres. | Mild processing conditions. | Complete removal of the unreacted crosslinking agent may be difficult due to possible toxicity. | 30–700 µm |  | 0.05–1.66% | Polymer concentration; crosslinking agent concentration; mixing speed and time; temperature; pH. | Microspheres [53,91,92,93,94]; microsphere-loaded core–shell carrier [50,95]. |
| Emulsion solvent diffusion method | An o/w emulsion is prepared by mixing organic solvent into a solution of CS with stabilizer under mechanical stirring, followed by high-pressure homogenization/ultra-sonication. Add a large amount of water to the emulsion to form particles. | High encapsulation efficiency of hydrophobic drugs. | High shear force involved in the process; use of organic solvent. | 0.1–45 µm |  | 2% | Solvent selection; emulsification conditions: stirring rate, emulsifying time and temperature. | Micro-/nanosphere [96,97]. |
| Spray-drying method | CS is first dissolved in an aqueous medium, and then the drug is dissolved or dispersed in the previous solution. Crosslinker is added to the polymeric solution. Particles are produced by atomization and subsequent solvent evaporation. | Low impact on the solubility of drug and polymer; simple, reproducible and easy to scale up. | Degradation due to high temperatures or/and high shear rates during atomization. | 0.2–60 µm |  | - | Feed composition and concentration; operation temperature; flow rate and pressure of the atomizing air; spray rate; drying time; type and concentration of the surfactant. | Micro-/nanosphere [54,98,99,100,101,102]. |
| Supercritical technique | Microspheres are fabricated by spraying a drug-loaded HCL/DMSO solution into supercritical carbon dioxide. | Small-sized particles (<3 µm); fast; cost-effective. | Rather broad particle size distribution. | 0.4–10 µm |  | - | Temperature and pressure of the supercritical fluid; solvent type and concentration; flow rate; nozzle geometry; antisolvent addition. | Nanosphere [56]; microsphere [103]. |
| Electrospraying | CS is dispersed/dissolved into a mixture of solvent and blend with drug solution/suspension. The conductive liquids are atomized under high voltage to form drug-encapsulated particles. The flow rate, voltage and distance between needle tip and collector are crucial process parameters. | Low production cost; narrow particle size distribution; easy-to-control surface properties and rapid preparation; high drug-loading efficiency; gentle conditions without use of harsh solvents. | Further investigation needed for upscaling; potential toxicity due to certain solvents. | 0.1–1.3 µm |  | - | Flow rate; solvent evaporation rate; collector distance; electrical conductivity; nature of polymer, solvent and molecules being used in the process. | Nanosphere [57,104,105]. |
| Reverse microemulsion/micellar method | Organic solvent (containing surfactant) is mixed with acidic CS solution to form reverse micelles. Then, drug conjugate and CS attach to the micelles via glutaraldehyde (crosslinker) to form nanoparticles. Residual solvent and surfactant and excess crosslinking agent need to be removed. | Ultrafine particle size (<100 nm); narrow particle size distribution. | Application of organic solvent; time-consuming preparation process; complex washing step. | 60–130 nm |  | 0.01–0.1% | Choice of surfactant and co-surfactant; type and concentration of oil phase; water-to-oil ratio; temperature and stirring speed; addition of crosslinking agents. | Nanosphere [59,60,106]. |
| Sieving method | A drug-loaded CS jelly mass is crosslinked and then manually passed through a sieve to obtain non-sticky particles. | Simple and commercially viable; easy scale-up; devoid of tedious processes; high drug loading. | Irregular particle shape. | 500–600 µm |   | - | Mesh size of the sieve; amplitude and frequency of vibration; duration of the sieving process; properties of the material. | Rod- or irregular-shaped microparticles [61]. |
| Solvent displacement/interfacial deposition method | Sub-microcapsule nanoemulsion coated with CS shell. | Suitable to encapsulate lipophilic drugs; rapid and easy operation; narrow size distribution; absence of shearing stress. | Use of organic solvents. | 130–500 nm |  | 0.1–0.33% | Polymer concentration; selection of solvent; selection of non-solvent; mixing rate; temperature; surfactant concentration; pH; addition rate. | Nanocapsule [107,108,109]. |
| Microfluidic technique | Dispersed phase and continuous phase are syringe-pumped onto microchannel of the microfluidic chip to obtain droplets, which are subsequently hardened by precipitation or crosslinking. | Well-controlled size; able to entrap hydrophilic and/or lipophilic molecules; controllable particles. | Low production rate; difficulties in mass production (scale-up) except parallelization; high cost. | 0.2–600 µm |    | - | Flow rate; viscosity; temperature; device geometry; electrical and magnetic fields. | Microcapsule [47,110,111]; multilayer particle [81,112,113]; microfiber [114]. |
3. Characteristics of Chitosan-Involved Particulate Carrier
4. Particulate Structure and Controlled Release Kinetics

5. Release Kinetics, Mechanisms and Modeling
6. Conclusive Remarks and Prospective Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tharanathan, R.N.; Kittur, F.S. Chitin—The Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-B.; He, Y.-S.; Li, S.-L.; Wang, Y.-Z. Chitin Whiskers: An Overview. Biomacromolecules 2012, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shit, S.C.; Shah, P.M. Edible Polymers: Challenges and Opportunities. J. Polym. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Martínez, J.P.; Falomir, M.P.; Gozalbo, D. Chitin: A Structural Biopolysaccharide with Multiple Applications. In eLS; American Cancer Society: Atlanta, GA, USA, 2014; ISBN 978-0-470-01590-2. [Google Scholar]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Gooday, G.W. The Ecology of Chitin Degradation. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Springer: Boston, MA, USA, 1990; pp. 387–430. [Google Scholar]
- Latańska, I.; Rosiak, P.; Paul, P.; Sujka, W.; Kolesińska, B.; Latańska, I.; Rosiak, P.; Paul, P.; Sujka, W.; Kolesińska, B. Modulating the Physicochemical Properties of Chitin and Chitosan as a Method of Obtaining New Biological Properties of Biodegradable Materials; IntechOpen: London, UK, 2021; ISBN 978-1-78984-425-2. [Google Scholar]
- Kim, S.-K. Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activities and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4398-1603-5. [Google Scholar]
- Shariatinia, Z. Pharmaceutical Applications of Chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Wu, Z.-X.; Yin, F.-W.; Song, S.; Li, A.; Zhu, B.-W.; Yu, L.-L. (Lucy). Chitosan and Derivatives: Bioactivities and Application in Foods. Annu. Rev. Food Sci. Technol. 2021, 12, 407–432. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of United Nations. The State of World Fisheries and Aquaculture 2014.; FAO: Rome, Italy, 2014. [Google Scholar]
- Yan, N.; Chen, X. Sustainability: Don’t Waste Seafood Waste. Nat. News 2015, 524, 155. [Google Scholar] [CrossRef]
- EUCHIS European Chitin Society-Euchis.Org. Available online: https://euchis.org/ (accessed on 22 February 2023).
- Riofrio, A.; Alcivar, T.; Baykara, H. Environmental and Economic Viability of Chitosan Production in Guayas-Ecuador: A Robust Investment and Life Cycle Analysis. ACS Omega 2021, 6, 23038–23051. [Google Scholar] [CrossRef]
- Alibaba Chitosan Price, 2023 Chitosan Price Manufacturers & Suppliers|Made-in-China.com. Available online: https://www.made-in-china.com/multi-search/Chitosan_Price/F2--CL_DM--PV_1356080000_19114_4374/1.html (accessed on 6 March 2023).
- SFly. PRODUCTEUR DE CHITINE, CHITOSAN-HERMETIA ILLUCENS. SFLY. Available online: http://sflyproteins.fr/ (accessed on 29 March 2019).
- Factors, F. Chitosan Industry Trends, Share, Value, Analysis & Forecast Report. 2022. Available online: https://www.globenewswire.com/en/news-release/2022/09/27/2523512/0/en/Demand-for-Global-Chitosan-Market-Size-to-Hit-26272-32-Mn-by-2028-Exhibit-a-CAGR-of-24-10-Chitosan-Industry-Trends-Share-Value-Analysis-Forecast-Report-by-Facts-Factors.html (accessed on 28 February 2023).
- Report Linker. Global Chitin and Chitosan Derivatives Market to Reach $24.9 Billion by 2030. Available online: https://www.globenewswire.com/news-release/2023/02/17/2610744/0/en/Global-Chitin-and-Chitosan-Derivatives-Market-to-Reach-24-9-Billion-by-2030.html (accessed on 6 March 2023).
- Munawar, A.M.; Syeda, J.; Wasan, K.; Wasan, E. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Jing, H.; Guo, Z.; Guo, W.; Yang, W.; Xu, P.; Zhang, X. Synthesis and Characterization of Folic Acid Modified Water-Soluble Chitosan Derivatives for Folate-Receptor-Mediated Targeting. Bioorg. Med. Chem. Lett. 2012, 22, 3418–3424. [Google Scholar] [CrossRef]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A Review on Chitosan and Chitosan-Based Bionanocomposites: Promising Material for Combatting Global Issues and Its Applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Thiolation of Chitosan. Attachment of Proteins via Thioether Formation. Biomacromolecules 2005, 6, 880–884. [Google Scholar] [CrossRef]
- Knight, D.K.; Shapka, S.N.; Amsden, B.G. Structure, Depolymerization, and Cytocompatibility Evaluation of Glycol Chitosan. J. Biomed. Mater. Res. Part A 2007, 83A, 787–798. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.H.; Kim, S.; Chung, H.; Choi, K.; Kwon, I.C.; Park, J.H.; Kim, Y.S.; Park, R.W.; Kim, I.S.; et al. Self-Assembled Nanoparticles of Bile Acid-Modified Glycol Chitosans and Their Applications for Cancer Therapy. Macromol. Res. 2005, 13, 167–175. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, Z.; Song, X.; Yu, B.; Bi, Y.; Chen, Q.; Zhao, D.; Xu, J.; Hou, S. Receptor Mediated Gene Delivery by Folate Conjugated N-Trimethyl Chitosan in Vitro. Int. J. Pharm. 2009, 382, 262–269. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, D.; Duan, C.; Jia, L.; Feng, F.; Liu, Y.; Wang, Y.; Hao, L.; Zhang, Q. Preparation and Characterizations of a Novel Deoxycholic Acid–O-Carboxymethylated Chitosan–Folic Acid Conjugates and Self-Aggregates. Carbohydr. Polym. 2011, 84, 1192–1200. [Google Scholar] [CrossRef]
- Li, D.-H.; Liu, L.-M.; Tian, K.-L.; Liu, J.-C.; Fan, X.-Q. Synthesis, Biodegradability and Cytotoxicity of Water-Soluble Isobutylchitosan. Carbohydr. Polym. 2007, 67, 40–45. [Google Scholar] [CrossRef]
- Engibaryan, L.G.; Chernukhina, A.I.; Gabrielyan, G.A.; Gal’braikh, L.S. Fabrication of New Water-Soluble Chitosan Derivatives. Fibre Chem. 2005, 37, 285–288. [Google Scholar] [CrossRef]
- Liu, S.-H.; Chen, R.-Y.; Chiang, M.-T. Effects and Mechanisms of Chitosan and Chitosan Oligosaccharide on Hepatic Lipogenesis and Lipid Peroxidation, Adipose Lipolysis, and Intestinal Lipid Absorption in Rats with High-Fat Diet-Induced Obesity. Int. J. Mol. Sci. 2021, 22, 1139. [Google Scholar] [CrossRef]
- Ait Hamdan, Y.; El Amerany, F.; Desbrières, J.; Aghrinane, A.; Oudadesse, H.; Rhazi, M. The Evolution of the Global COVID-19 Epidemic in Morocco and Understanding the Different Therapeutic Approaches of Chitosan in the Control of the Pandemic. Polym. Bull. 2022, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N.; El-Kemary, M.A. Silymarin/Curcumin Loaded Albumin Nanoparticles Coated by Chitosan as Muco-Inhalable Delivery System Observing Anti-Inflammatory and Anti COVID-19 Characterizations in Oleic Acid Triggered Lung Injury and in Vitro COVID-19 Experiment. Int. J. Biol. Macromol. 2022, 198, 101–110. [Google Scholar] [CrossRef]
- Landauer, J.; Kuhn, M.; Nasato, D.S.; Foerst, P.; Briesen, H. Particle Shape Matters-Using 3D Printed Particles to Investigate Fundamental Particle and Packing Properties. Powder Technol. 2020, 361, 711–718. [Google Scholar] [CrossRef]
- Dasgupta, S.; Auth, T.; Gompper, G. Shape and Orientation Matter for the Cellular Uptake of Nonspherical Particles. Nano Lett. 2014, 14, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate Gel Particles-A Review of Production Techniques and Physical Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Animasaun, I.L.; Yook, S.-J.; Al-Mdallal, Q.M.; Shah, N.A.; Faisal, M. Insight into the Motion of Water Conveying Three Kinds of Nanoparticles Shapes on a Horizontal Surface: Significance of Thermo-Migration and Brownian Motion. Surf. Interfaces 2022, 30, 101854. [Google Scholar] [CrossRef]
- Chen, M.-M.; Cao, H.; Liu, Y.-Y.; Liu, Y.; Song, F.-F.; Chen, J.-D.; Zhang, Q.-Q.; Yang, W.-Z. Sequential Delivery of Chlorhexidine Acetate and BFGF from PLGA-Glycol Chitosan Core-Shell Microspheres. Colloids Surf. B Biointerfaces 2017, 151, 189–195. [Google Scholar] [CrossRef]
- Tsai, M.-S.; Li, M.J. A Novel Process to Prepare a Hollow Silica Sphere via Chitosan-Polyacrylic Acid (CS-PAA) Template. J. Non-Cryst. Solids 2006, 352, 2829–2833. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for Biorelated Polymers and Applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 34. [Google Scholar] [CrossRef]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric Nanoparticles, Nanospheres and Nanocapsules, for Cutaneous Applications. Drug Target Insights 2007, 2, 117739280700200. [Google Scholar] [CrossRef]
- Couvreur, P.; Barratt, G.; Fattal, E.; Vauthier, C. Nanocapsule Technology: A Review. Crit. Rev. Ther. Drug Carr. Syst. 2002, 19. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Guterres, S.S.; Freitas, L.D.L.; Pohlmann, A.R. Physicochemical Characterization and Stability of the Polymeric Nanoparticle Systems for Drug Administration. Quím. Nova 2003, 26, 726–737. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chang, C.-H.; Wu, Y.-S.; Hsu, Y.-M.; Chiou, S.-F.; Chen, Y.-J. Development of PH-Responsive Chitosan/Heparin Nanoparticles for Stomach-Specific Anti-Helicobacter Pylori Therapy. Biomaterials 2009, 30, 3332–3342. [Google Scholar] [CrossRef]
- Arozal, W.; Louisa, M.; Rahmat, D.; Chendrana, P.; Sandhiutami, N.M.D. Development, Characterization and Pharmacokinetic Profile of Chitosan-Sodium Tripolyphosphate Nanoparticles Based Drug Delivery Systems for Curcumin. Adv. Pharm. Bull. 2021, 11, 77–85. [Google Scholar] [CrossRef]
- Gooneh-Farahani, S.; Naghib, S.M.; Naimi-Jamal, M.R. A Novel and Inexpensive Method Based on Modified Ionic Gelation for PH-Responsive Controlled Drug Release of Homogeneously Distributed Chitosan Nanoparticles with a High Encapsulation Efficiency. Fibers Polym. 2020, 21, 1917–1926. [Google Scholar] [CrossRef]
- Xun, X.-M.; Zhang, Z.-A.; Yuan, Z.-X.; Tuhong, K.; Yan, C.-H.; Zhan, Y.-F.; Kang, G.-P.; Wu, Q.-Y.; Wang, J. Novel Caffeic Acid Grafted Chitosan-Sodium Alginate Microcapsules Generated by Microfluidic Technique for the Encapsulation of Bioactive Peptides from Silkworm Pupae. Sustain. Chem. Pharm. 2023, 32, 100974. [Google Scholar] [CrossRef]
- Park, J.W.; Park, K.H.; Seo, S. Natural Polyelectrolyte Complex-based PH-dependent Delivery Carriers Using Alginate and Chitosan. J. Appl. Polym. Sci. 2019, 136, 48143. [Google Scholar] [CrossRef]
- Nazlı, A.B.; Açıkel, Y.S. Loading of Cancer Drug Resveratrol to PH-Sensitive, Smart, Alginate-Chitosan Hydrogels and Investigation of Controlled Release Kinetics. J. Drug Deliv. Sci. Technol. 2019, 53, 101199. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, Z.; Li, Y.; Zhu, S.; Ma, J.; Li, W.; Gao, G. Development and Characterization of Cores–Shell Poly(Lactide-Co-Glycolide)-Chitosan Microparticles for Sustained Release of GDNF. Colloids Surf. B Biointerfaces 2017, 159, 791–799. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M.; Argüelles-Monal, W. Microencapsulation of Astaxanthin in a Chitosan Matrix. Carbohydr. Polym. 2004, 56, 41–45. [Google Scholar] [CrossRef]
- Huang, W.F.; Tsui, C.P.; Tang, C.Y.; Yang, M.; Gu, L. Surface Charge Switchable and PH-Responsive Chitosan/Polymer Core-Shell Composite Nanoparticles for Drug Delivery Application. Compos. Part B Eng. 2017, 121, 83–91. [Google Scholar] [CrossRef]
- Dozie-Nwachukwu, S.O.; Danyuo, Y.; Obayemi, J.D.; Odusanya, O.S.; Malatesta, K.; Soboyejo, W.O. Extraction and Encapsulation of Prodigiosin in Chitosan Microspheres for Targeted Drug Delivery. Mater. Sci. Eng. C 2017, 71, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Budincic, J.M.; Petrovic, L.; Dekic, L.; Fraj, J.; Bucko, S.; Katona, J.; Spasojevic, L. Study of Vitamin E Microencapsulation and Controlled Release from Chitosan/Sodium Lauryl Ether Sulfate Microcapsules. Carbohydr. Polym. 2021, 251, 116988. [Google Scholar] [CrossRef]
- Kurakula, M.; Raghavendra Naveen, N. Electrospraying: A Facile Technology Unfolding the Chitosan Based Drug Delivery and Biomedical Applications. Eur. Polym. J. 2021, 147, 110326. [Google Scholar] [CrossRef]
- Hijazi, N.; Rodier, E.; Letourneau, J.-J.; Louati, H.; Sauceau, M.; Le Moigne, N.; Benezet, J.-C.; Fages, J. Chitosan Nanoparticles Generation Using CO2 Assisted Processes. J. Supercrit. Fluids 2014, 95, 118–128. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Phan, V.H.G.; Lee, D.S.; Thambi, T.; Huynh, D.P. Bioresorbable PH- and Temperature-Responsive Injectable Hydrogels-Incorporating Electrosprayed Particles for the Sustained Release of Insulin. Polym. Degrad. Stab. 2019, 162, 36–46. [Google Scholar] [CrossRef]
- Hiroyuki, T.; Hideki, I.; Yoshinobu, F. Chitosan-Gadopentetic Acid Complex Nanoparticles for Gadolinium Neutron-Capture Therapy of Cancer: Preparation by Novel Emulsion-Droplet Coalescence Technique and Characterization. Pharm. Res. 1999, 16, 1830–1835. [Google Scholar] [CrossRef]
- Sheikholeslami, Z.S.; Salimi-Kenari, H.; Imani, M.; Atai, M.; Nodehi, A. Exploring the Effect of Formulation Parameters on the Particle Size of Carboxymethyl Chitosan Nanoparticles Prepared via Reverse Micellar Crosslinking. J. Microencapsul. 2017, 34, 270–279. [Google Scholar] [CrossRef]
- Mitra, S.; Gaur, U.; Ghosh, P.C.; Maitra, A.N. Tumour Targeted Delivery of Encapsulated Dextran–Doxorubicin Conjugate Using Chitosan Nanoparticles as Carrier. J. Control. Release 2001, 74, 317–323. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Aminabhavi, T.M. Controlled Release of Clozapine through Chitosan Microparticles Prepared by a Novel Method. J. Control. Release 2004, 96, 245–259. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Towards Better Modeling of Chitosan Nanoparticles Production: Screening Different Factors and Comparing Two Experimental Designs. Int. J. Biol. Macromol. 2014, 64, 334–340. [Google Scholar] [CrossRef]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters Influencing the Size of Chitosan-TPP Nano- and Microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef]
- Thakur, A.; Taranjit. Preparation of Chitosan Nanoparticles: A Study of Influencing Factors. In Proceedings of the International Conference On Advances in Condensed And Nano Materials (ICACNM-2011), Chandigarh, India, 23–26 February 2011; AIP Conference Proceedings: College Park, MD, USA, 2011; pp. 299–300.
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil Encapsulation Techniques Using Alginate as Encapsulating Agent: Applications and Drawbacks. J. Microencapsul. 2017, 34, 754–771. [Google Scholar] [CrossRef]
- Lee, Y.; Ji, Y.R.; Lee, S.; Choi, M.-J.; Cho, Y. Microencapsulation of Probiotic Lactobacillus Acidophilus KBL409 by Extrusion Technology to Enhance Survival under Simulated Intestinal and Freeze-Drying Conditions. J. Microbiol. Biotechnol. 2019, 29, 721–730. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel Hydrophilic Chitosan-Polyethylene Oxide Nanoparticles as Protein Carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation Mechanism of Monodisperse, Low Molecular Weight Chitosan Nanoparticles by Ionic Gelation Technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the Antifungal Activity of Clove Essential Oil Encapsulated by Chitosan Nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles for Transdermal Delivery. AAPS PharmSciTech 2019, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.N.Q.; Bach, L.G.; Nguyen, T.D.; Le, N.T.H. Efficient Method for Preparation of Rutin Nanosuspension Using Chitosan and Sodium Tripolyphosphate Crosslinker. J. Nanosci. Nanotechnol. 2019, 19, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Bin-Jumah, M.; Gilani, S.J.; Jahangir, M.A.; Zafar, A.; Alshehri, S.; Yasir, M.; Kala, C.; Taleuzzaman, M.; Imam, S.S. Clarithromycin-Loaded Ocular Chitosan Nanoparticle: Formulation, Optimization, Characterization, Ocular Irritation, and Antimicrobial Activity. Available online: https://www.dovepress.com/clarithromycin-loaded-ocular-chitosan-nanoparticle-formulation-optimiz-peer-reviewed-fulltext-article-IJN (accessed on 15 January 2021).
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Upadhyay, N.; Singh, P.; Sharma, S.; Dubey, N.K. Encapsulation in Chitosan-Based Nanomatrix as an Efficient Green Technology to Boost the Antimicrobial, Antioxidant and in Situ Efficacy of Coriandrum Sativum Essential Oil. Int. J. Biol. Macromol. 2019, 133, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, X.; Ding, Y.; Ge, H.; Yuan, Y.; Yang, C. Synthesis and Characterization of Chitosan–Poly(Acrylic Acid) Nanoparticles. Biomaterials 2002, 23, 3193–3201. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, X.; Shi, S.; Zheng, X.; Guo, G.; Wei, Y.; Qian, Z. Preparation of Alginate Coated Chitosan Microparticles for Vaccine Delivery. BMC Biotechnol. 2008, 8, 89. [Google Scholar] [CrossRef]
- Liu, F.; Liu, L.; Li, X.; Zhang, Q. Preparation of Chitosan–Hyaluronate Double-Walled Microspheres by Emulsification-Coacervation Method. J. Mater. Sci. Mater. Med. 2007, 18, 2215–2224. [Google Scholar] [CrossRef]
- Rathore, P. Formulation Development, in Vitro and in Vivo Evaluation of Chitosan Engineered Nanoparticles for Ocular Delivery of Insulin. RSC Adv. 2020, 10, 43629–43639. [Google Scholar] [CrossRef]
- Saraf, S.; Jain, S.; Sahoo, R.N.; Mallick, S. Lipopolysaccharide Derived Alginate Coated Hepatitis B Antigen Loaded Chitosan Nanoparticles for Oral Mucosal Immunization. Int. J. Biol. Macromol. 2020, 154, 466–476. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Li, H.; Wei, C.; Wu, C. Onion-Structure Bionic Hydrogel Capsules Based on Chitosan for Regulating Doxorubicin Release. Carbohydr. Polym. 2019, 209, 152–160. [Google Scholar] [CrossRef]
- Rodkate, N.; Rutnakornpituk, M. Multi-Responsive Magnetic Microsphere of Poly(N-Isopropylacrylamide)/Carboxymethylchitosan Hydrogel for Drug Controlled Release. Carbohydr. Polym. 2016, 151, 251–259. [Google Scholar] [CrossRef]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate Coated Chitosan Core-Shell Nanoparticles for Efficient Oral Delivery of Naringenin in Diabetic Animals—An in Vitro and in Vivo Approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef]
- Soe, Z.C.; Poudel, B.K.; Nguyen, H.T.; Thapa, R.K.; Ou, W.; Gautam, M.; Poudel, K.; Jin, S.G.; Jeong, J.-H.; Ku, S.K.; et al. Folate-Targeted Nanostructured Chitosan/Chondroitin Sulfate Complex Carriers for Enhanced Delivery of Bortezomib to Colorectal Cancer Cells. Asian J. Pharm. Sci. 2019, 14, 40–51. [Google Scholar] [CrossRef]
- Chaiyasan, W.; Srinivas, S.P.; Tiyaboonchai, W. Crosslinked Chitosan-Dextran Sulfate Nanoparticle for Improved Topical Ocular Drug Delivery. Mol. Vis. 2015, 21, 1224–1234. [Google Scholar]
- Mukhopadhyay, P.; Chakraborty, S.; Bhattacharya, S.; Mishra, R.; Kundu, P.P. PH-Sensitive Chitosan/Alginate Core-Shell Nanoparticles for Efficient and Safe Oral Insulin Delivery. Int. J. Biol. Macromol. 2015, 72, 640–648. [Google Scholar] [CrossRef]
- Özbaş-Turan, S.; Akbuǧa, J.; Aral, C. Controlled Release of Interleukin-2 from Chitosan Microspheres. J. Pharm. Sci. 2002, 91, 1245–1251. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, V.; Singh, P.P.; Kujur, A.; Prakash, B. Fabrication of Volatile Compounds Loaded-Chitosan Biopolymer Nanoparticles: Optimization, Characterization and Assessment against Aspergillus Flavus and Aflatoxin B1 Contamination. Int. J. Biol. Macromol. 2020, 165, 1507–1518. [Google Scholar] [CrossRef]
- Lam, P.-L.; Lee, K.K.-H.; Ho, Y.-W.; Wong, R.S.-M.; Tong, S.-W.; Cheng, C.-H.; Lam, K.-H.; Tang, J.C.-O.; Bian, Z.-X.; Gambari, R.; et al. The Development of Chitosan Based Microcapsules as Delivery Vehicles for Orally Administered Daunorubicin. RSC Adv. 2014, 4, 14109–14114. [Google Scholar] [CrossRef]
- Lim, L.Y.; Wan, L.S.C.; Thai, P.Y. Chitosan Microspheres Prepared by Emulsification and Ionotropic Gelation. Drug Dev. Ind. Pharm. 1997, 23, 981–985. [Google Scholar] [CrossRef]
- Biró, E.; Németh, A.S.; Feczkó, T.; Tóth, J.; Sisak, C.; Gyenis, J. Three-Step Experimental Design to Determine the Effect of Process Parameters on the Size of Chitosan Microspheres. Chem. Eng. Process. Process Intensif. 2009, 48, 771–779. [Google Scholar] [CrossRef]
- Patil, S.B.; Sawant, K.K. Chitosan Microspheres as a Delivery System for Nasal Insufflation. Colloids Surf. B Biointerfaces 2011, 84, 384–389. [Google Scholar] [CrossRef]
- Dhawan, S.; Singla, A.K.; Sinha, V.R. Evaluation of Mucoadhesive Properties of Chitosan Microspheres Prepared by Different Methods. AAPS PharmSciTech 2004, 5, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Nayak, U.Y.; Gopal, S.; Mutalik, S.; Ranjith, A.K.; Reddy, M.S.; Gupta, P.; Udupa, N. Glutaraldehyde Cross-Linked Chitosan Microspheres for Controlled Delivery of Zidovudine. J. Microencapsul. 2009, 26, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Abulaihaiti, M.; Wu, X.-W.; Qiao, L.; Lv, H.-L.; Zhang, H.-W.; Aduwayi, N.; Wang, Y.-J.; Wang, X.-C.; Peng, X.-Y. Efficacy of Albendazole-Chitosan Microsphere-Based Treatment for Alveolar Echinococcosis in Mice. PLoS Negl. Trop. Dis. 2015, 9, e0003950. [Google Scholar] [CrossRef] [PubMed]
- El-Shabouri, M.H. Positively Charged Nanoparticles for Improving the Oral Bioavailability of Cyclosporin-A. Int. J. Pharm. 2002, 249, 101–108. [Google Scholar] [CrossRef]
- Niwa, T.; Takeuchi, H.; Hino, T.; Kunou, N.; Kawashima, Y. Preparations of Biodegradable Nanospheres of Water-Soluble and Insoluble Drugs with D,L-Lactide/Glycolide Copolymer by a Novel Spontaneous Emulsification Solvent Diffusion Method, and the Drug Release Behavior. J. Control. Release 1993, 25, 89–98. [Google Scholar] [CrossRef]
- Liu, W.; Wu, W.D.; Selomulya, C.; Chen, X.D. Uniform Chitosan Microparticles Prepared by a Novel Spray-Drying Technique. Int. J. Chem. Eng. 2011, 2011, 267218. [Google Scholar] [CrossRef]
- Ngan, L.T.K.; Wang, S.-L.; Hiep, Đ.M.; Luong, P.M.; Vui, N.T.; Đinh, T.M.; Dzung, N.A. Preparation of Chitosan Nanoparticles by Spray Drying, and Their Antibacterial Activity. Res. Chem. Intermed. 2014, 40, 2165–2175. [Google Scholar] [CrossRef]
- Acosta, N.; Sánchez, E.; Calderón, L.; Cordoba-Diaz, M.; Cordoba-Diaz, D.; Dom, S.; Heras, Á. Physical Stability Studies of Semi-Solid Formulations from Natural Compounds Loaded with Chitosan Microspheres. Mar. Drugs 2015, 13, 5901–5919. [Google Scholar] [CrossRef]
- Aranaz, I.; Paños, I.; Peniche, C.; Heras, Á.; Acosta, N. Chitosan Spray-Dried Microparticles for Controlled Delivery of Venlafaxine Hydrochloride. Molecules 2017, 22, 1980. [Google Scholar] [CrossRef]
- Khlibsuwan, R.; Siepmann, F.; Siepmann, J.; Pongjanyakul, T. Chitosan-Clay Nanocomposite Microparticles for Controlled Drug Delivery: Effects of the MAS Content and TPP Crosslinking. J. Drug Deliv. Sci. Technol. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Amidi, M.; Pellikaan, H.C.; de Boer, A.H.; Crommelin, D.J.A.; Hennink, W.E.; Jiskoot, W. Preparation and Physicochemical Characterization of Supercritically Dried Insulin-Loaded Microparticles for Pulmonary Delivery. Eur. J. Pharm. Biopharm. 2008, 68, 191–200. [Google Scholar] [CrossRef]
- Moreno, J.A.S.; Mendes, A.C.; Stephansen, K.; Engwer, C.; Goycoolea, F.M.; Boisen, A.; Nielsen, L.H.; Chronakis, I.S. Development of Electrosprayed Mucoadhesive Chitosan Microparticles. Carbohydr. Polym. 2018, 190, 240–247. [Google Scholar] [CrossRef]
- Sreekumar, S.; Lemke, P.; Moerschbacher, B.M.; Torres-Giner, S.; Lagaron, J.M. Preparation and Optimization of Submicron Chitosan Capsules by Water-Based Electrospraying for Food and Bioactive Packaging Applications. Food Addit. Contam. Part A 2017, 34, 1795–1806. [Google Scholar] [CrossRef]
- Cui, C.; Gan, L.; Lan, X.; Li, J.; Zhang, C.; Li, F.; Wang, C.; Yuan, C. Development of Sustainable Carrier in Thermosensitive Hydrogel Based on Chitosan/Alginate Nanoparticles for in Situ Delivery System. Polym. Compos. 2019, 40, 2187–2196. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Development of Positively Charged Colloidal Drug Carriers: Chitosan-Coated Polyester Nanocapsules and Submicron-Emulsions. Colloid Polym. Sci. 1997, 275, 46–53. [Google Scholar] [CrossRef]
- Hoffmann, S.; Gorzelanny, C.; Moerschbacher, B.; Goycoolea, F. Physicochemical Characterization of FRET-Labelled Chitosan Nanocapsules and Model Degradation Studies. Nanomaterials 2018, 8, 846. [Google Scholar] [CrossRef]
- Prego, C.; Fabre, M.; Torres, D.; Alonso, M.J. Efficacy and Mechanism of Action of Chitosan Nanocapsules for Oral Peptide Delivery. Pharm. Res. 2006, 23, 549–556. [Google Scholar] [CrossRef]
- Yang, X.-L.; Ju, X.-J.; Mu, X.-T.; Wang, W.; Xie, R.; Liu, Z.; Chu, L.-Y. Core–Shell Chitosan Microcapsules for Programmed Sequential Drug Release. ACS Appl. Mater. Interfaces 2016, 8, 10524–10534. [Google Scholar] [CrossRef]
- Huang, K.-S.; Yang, C.-H.; Wang, Y.-C.; Wang, W.-T.; Lu, Y.-Y. Microfluidic Synthesis of Vinblastine-Loaded Multifunctional Particles for Magnetically Responsive Controlled Drug Release. Pharmaceutics 2019, 11, 212. [Google Scholar] [CrossRef]
- Mou, C.-L.; Wang, W.; Ju, X.-J.; Xie, R.; Liu, Z.; Chu, L.-Y. Dual-Responsive Microcarriers with Sphere-in-Capsule Structures for Co-Encapsulation and Sequential Release. J. Taiwan Inst. Chem. Eng. 2019, 98, 63–69. [Google Scholar] [CrossRef]
- Ling, K.; Wu, H.; Neish, A.S.; Champion, J.A. Alginate/Chitosan Microparticles for Gastric Passage and Intestinal Release of Therapeutic Protein Nanoparticles. J. Control. Release 2019, 295, 174–186. [Google Scholar] [CrossRef] [PubMed]
- He, X.-H.; Wang, W.; Deng, K.; Xie, R.; Ju, X.-J.; Liu, Z.; Chu, L.-Y. Microfluidic Fabrication of Chitosan Microfibers with Controllable Internals from Tubular to Peapod-like Structures. RSC Adv. 2015, 5, 928–936. [Google Scholar] [CrossRef]
- Rao, A.; Schoenenberger, M.; Gnecco, E.; Glatzel, T.; Meyer, E.; Brändlin, D.; Scandella, L. Characterization of Nanoparticles Using Atomic Force Microscopy. J. Phys. Conf. Ser. 2007, 61, 971. [Google Scholar] [CrossRef]
- Otles, S.; Ozyurt, V.H. Electron Mıcroscopy Technıques. In Microstructure of Dairy Products; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 51–66. [Google Scholar]
- Pei, Y.; Hinchliffe, B.A.; Minelli, C. Measurement of the Size Distribution of Multimodal Colloidal Systems by Laser Diffraction. ACS Omega 2021, 6, 14049–14058. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; Courier Corporation: Chelmsford, MA, USA, 2000. [Google Scholar]
- Agarwal, R.; Jurney, P.; Raythatha, M.; Singh, V.; Sreenivasan, S.V.; Shi, L.; Roy, K. Effect of Shape, Size, and Aspect Ratio on Nanoparticle Penetration and Distribution inside Solid Tissues Using 3D Spheroid Models. Adv. Healthc. Mater. 2015, 4, 2269–2280. [Google Scholar] [CrossRef]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape Effects of Filaments versus Spherical Particles in Flow and Drug Delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef]
- Boulanger, G.; Andujar, P.; Pairon, J.-C.; Billon-Galland, M.-A.; Dion, C.; Dumortier, P.; Brochard, P.; Sobaszek, A.; Bartsch, P.; Paris, C.; et al. Quantification of Short and Long Asbestos Fibers to Assess Asbestos Exposure: A Review of Fiber Size Toxicity. Environ. Health 2014, 13, 59. [Google Scholar] [CrossRef]
- Chandra, J.; George, N.; Narayanankutty, S.K. Isolation and Characterization of Cellulose Nanofibrils from Arecanut Husk Fibre. Carbohydr. Polym. 2016, 142, 158–166. [Google Scholar] [CrossRef]
- Ouf, F.-X.; Bourrous, S.; Vallières, C.; Yon, J.; Lintis, L. Specific Surface Area of Combustion Emitted Particles: Impact of Primary Particle Diameter and Organic Content. J. Aerosol Sci. 2019, 137, 105436. [Google Scholar] [CrossRef]
- Li, T.; Senesi, A.J.; Lee, B. Small Angle X-Ray Scattering for Nanoparticle Research. Chem. Rev. 2016, 116, 11128–11180. [Google Scholar] [CrossRef]
- Svergun, D.I.; Koch, M.H.J. Small-Angle Scattering Studies of Biological Macromolecules in Solution. Rep. Prog. Phys. 2003, 66, 1735. [Google Scholar] [CrossRef]
- Klose, D.; Siepmann, F.; Elkharraz, K.; Krenzlin, S.; Siepmann, J. How Porosity and Size Affect the Drug Release Mechanisms from PLGA-Based Microparticles. Int. J. Pharm. 2006, 314, 198–206. [Google Scholar] [CrossRef]
- Mi, F.-L.; Lin, Y.-M.; Wu, Y.-B.; Shyu, S.-S.; Tsai, Y.-H. Chitin/PLGA Blend Microspheres as a Biodegradable Drug-Delivery System: Phase-Separation, Degradation and Release Behavior. Biomaterials 2002, 23, 3257–3267. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Kundu, P.P. Chitosan-Graft-PAMAM–Alginate Core–Shell Nanoparticles: A Safe and Promising Oral Insulin Carrier in an Animal Model. RSC Adv. 2015, 5, 93995–94007. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y. Hollow Nanometer-Sized Polypyrrole Capsules with Controllable Shell Thickness Synthesized in the Presence of Chitosan. Polymer 2005, 46, 5324–5328. [Google Scholar] [CrossRef]
- Lamprecht, A.; Schäfer, U.F.; Lehr, C.-M. Characterization of Microcapsules by Confocal Laser Scanning Microscopy: Structure, Capsule Wall Composition and Encapsulation Rate. Eur. J. Pharm. Biopharm. 2000, 49, 1–9. [Google Scholar] [CrossRef]
- Liu, C.; Pei, R.; Peltonen, L.; Heinonen, M. Assembling of the Interfacial Layer Affects the Physical and Oxidative Stability of Faba Bean Protein-Stabilized Oil-in-Water Emulsions with Chitosan. Food Hydrocoll. 2020, 102, 105614. [Google Scholar] [CrossRef]
- Han, Y.; Tong, W.; Zhang, Y.; Gao, C. Fabrication of Chitosan Single-Component Microcapsules With a Micrometer-Thick and Layered Wall Structure by Stepwise Core-Mediated Precipitation. Macromol. Rapid Commun. 2012, 33, 326–331. [Google Scholar] [CrossRef]
- Arzumanyan, G.; Linnik, D.; Mamatkulov, K.; Vorobyeva, M.; Korsun, A.; Glasunova, V.; Jevremović, A. Synthesis of NaYF4:Yb,Er@SiO2@Ag Core-Shell Nanoparticles for Plasmon-Enhanced Upconversion Luminescence in Bio-Applications. Ann. Biomed. Sci. Eng. 2019, 3, 013–019. [Google Scholar] [CrossRef]
- Grassi, M.; Grassi, G. Mathematical Modelling and Controlled Drug Delivery: Matrix Systems. Curr. Drug Deliv. 2005, 2, 97–116. [Google Scholar] [CrossRef]
- Feng, S.-S.; Ruan, G.; Li, Q.-T. Fabrication and Characterizations of a Novel Drug Delivery Device Liposomes-in-Microsphere (LIM). Biomaterials 2004, 25, 5181–5189. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Mu, B.; Liu, P. Stimuli-Responsive Multilayer Chitosan Hollow Microspheres via Layer-by-Layer Assembly. Colloids Surf. B Biointerfaces 2011, 83, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Williams, G.R.; Wu, J.; Wu, J.; Zhang, X.; Zheng, H.; Li, S.; Zhu, L.-M. A Novel Chitosan-Based Nanomedicine for Multi-Drug Resistant Breast Cancer Therapy. Chem. Eng. J. 2019, 369, 134–149. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug Release Study of the Chitosan-Based Nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef]
- Wagner, J.G. Interpretation of Percent Dissolved-Time Plots Derived from In Vitro Testing of Conventional Tablets and Capsules. J. Pharm. Sci. 1969, 58, 1253–1257. [Google Scholar] [CrossRef]
- Gibaldi, M.; Feldman, S. Establishment of Sink Conditions in Dissolution Rate Determinations. Theoretical Considerations and Application to Nondisintegrating Dosage Forms. J. Pharm. Sci. 1967, 56, 1238–1242. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of Sustained-Action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Kosmidis, K.; Argyrakis, P.; Macheras, P. Fractal Kinetics in Drug Release from Finite Fractal Matrices. J. Chem. Phys. 2003, 119, 6373–6377. [Google Scholar] [CrossRef]
- Hixson, A.W.; Crowell, J.H. Dependence of Reaction Velocity upon Surface and Agitation. Ind. Eng. Chem. 1931, 23, 923–931. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Kopcha, M.; Lordi, N.G.; Tojo, K.J. Evaluation of Release from Selected Thermosoftening Vehicles. J. Pharm. Pharmacol. 1991, 43, 382–387. [Google Scholar] [CrossRef]
- Baker, R.; Lonsdale, H. Controlled Release: Mechanisms and Rates. Control. Release Biol. Act. Agents 1974, 15. [Google Scholar]
- Shukla, A.J.; Price, J.C. Effect of Drug (Core) Particle Size on the Dissolution of Theophylline from Microspheres Made from Low Molecular Weight Cellulose Acetate Propionate. Pharm. Res. 1989, 6, 418–421. [Google Scholar] [CrossRef]
- Shukla, A.J.; Price, J.C. Effect of Drug Loading and Molecular Weight of Cellulose Acetate Propionate on the Release Characteristics of Theophylline Microspheres. Pharm. Res. 1991, 8, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Hopfenberg, H.B. Controlled Release from Erodible Slabs, Cylinders, and Spheres. In Controlled Release Polymeric Formulations; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1976; Volume 33, pp. 26–32. ISBN 978-0-8412-0341-9. [Google Scholar]
- Tsirigotis-Maniecka, M.; Szyk-Warszyńska, L.; Maniecki, Ł.; Szczęsna, W.; Warszyński, P.; Wilk, K.A. Tailoring the Composition of Hydrogel Particles for the Controlled Delivery of Phytopharmaceuticals. Eur. Polym. J. 2021, 151, 110429. [Google Scholar] [CrossRef]
- Bugatti, V.; Vertuccio, L.; Zara, S.; Fancello, F.; Scanu, B.; Gorrasi, G. Green Pesticides Based on Cinnamate Anion Incorporated in Layered Double Hydroxides and Dispersed in Pectin Matrix. Carbohydr. Polym. 2019, 209, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Jardim, K.V.; Palomec-Garfias, A.F.; Araújo, M.V.; Márquez-Beltrán, C.; Bakuzis, A.F.; Moya, S.E.; Parize, A.L.; Sousa, M.H. Remotely Triggered Curcumin Release from Stimuli-Responsive Magneto-Polymeric Layer-by-Layer Engineered Nanoplatforms. J. Appl. Polym. Sci. 2022, 139, 52200. [Google Scholar] [CrossRef]
- Liu, C.; Desai, K.G.H.; Tang, X.; Chen, X. Drug Release Kinetics of Spray-Dried Chitosan Microspheres. Dry. Technol. 2006, 24, 769–776. [Google Scholar] [CrossRef]
- Dahan, W.M.; Mohammad, F.; Ezzat, A.O.; Atta, A.M.; Al-Tilasi, H.H.; Al-Lohedan, H.A. Influence of Amidation on the Release Profiles of Insulin Drug from Chitosan-Based Matrices. Coatings 2022, 12, 465. [Google Scholar] [CrossRef]
- Thai, H.; Thuy Nguyen, C.; Thi Thach, L.; Thi Tran, M.; Duc Mai, H.; Thi Thu Nguyen, T.; Duc Le, G.; Van Can, M.; Dai Tran, L.; Long Bach, G.; et al. Characterization of Chitosan/Alginate/Lovastatin Nanoparticles and Investigation of Their Toxic Effects in Vitro and in Vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef]
- Whitehead, F.A.; Kasapis, S. Modelling the Mechanism and Kinetics of Ascorbic Acid Diffusion in Genipin-Crosslinked Gelatin and Chitosan Networks at Distinct PH. Food Biosci. 2022, 46, 101579. [Google Scholar] [CrossRef]
- Tapia, C.; Escobar, Z.; Costa, E.; Sapag-Hagar, J.; Valenzuela, F.; Basualto, C.; Nella Gai, M.; Yazdani-Pedram, M. Comparative Studies on Polyelectrolyte Complexes and Mixtures of Chitosan–Alginate and Chitosan–Carrageenan as Prolonged Diltiazem Clorhydrate Release Systems. Eur. J. Pharm. Biopharm. 2004, 57, 65–75. [Google Scholar] [CrossRef]
- Liu, H.; Gong, L.; Lu, S.; Wang, H.; Fan, W.; Yang, C. Three Core-Shell Polymersomes for Targeted Doxorubicin Delivery: Sustained and Acidic Release. J. Drug Deliv. Sci. Technol. 2021, 61, 102293. [Google Scholar] [CrossRef]
- Yan, S.; Rao, S.; Zhu, J.; Wang, Z.; Zhang, Y.; Duan, Y.; Chen, X.; Yin, J. Nanoporous Multilayer Poly(l-Glutamic Acid)/Chitosan Microcapsules for Drug Delivery. Int. J. Pharm. 2012, 427, 443–451. [Google Scholar] [CrossRef]
- Zhou, Y.; Chu, J.S.; Wu, X.Y. Theoretical Analysis of Drug Release into a Finite Medium from Sphere Ensembles with Various Size and Concentration Distributions. Eur. J. Pharm. Sci. 2004, 22, 251–259. [Google Scholar] [CrossRef]
- Spiridonova, T.I.; Tverdokhlebov, S.I.; Anissimov, Y.G. Investigation of the Size Distribution for Diffusion-Controlled Drug Release From Drug Delivery Systems of Various Geometries. J. Pharm. Sci. 2019, 108, 2690–2697. [Google Scholar] [CrossRef]
- Teimouri, S.; Dekiwadia, C.; Kasapis, S. Decoupling Diffusion and Macromolecular Relaxation in the Release of Vitamin B6 from Genipin-Crosslinked Whey Protein Networks. Food Chem. 2021, 346, 128886. [Google Scholar] [CrossRef]






| Product | Industrial Production (106 Tons Per Year) | Price (USD Per Ton) |
|---|---|---|
| Dried shrimp shells | 6–8 | 100–120 [14,15] |
| Chitin | 0.02–0.04 i | 6000–40,000 |
| Chitosan | <0.2 ii | 15,000–160,000 iii |
| Mechanism (s) | Description of Systems | Model | Equation(s) | References |
|---|---|---|---|---|
| Diffusion | Crosslinked CS-dextran sulfate nanoparticle | Higuchi | (2) | [85] |
| Crosslinked CS microspheres | Korsmeyer–Peppas/Higuchi | (3)(2) | [94] | |
| Spray-Dried CS Microspheres | Higuchi/Korsmeyer | (2)(3) | [155] | |
| CS hydrogel | Zero-order kinetic | (1) | [156] | |
| Fatty acid-grafted CS hydrogel | Higuchi | (2) | ||
| CS-LLA i | Hixson | (4) | ||
| CS–alginate nanoparticles | Korsmeyer–Peppas | (3) | [157] | |
| Diffusion and relaxation | CS–genipin matrices | Peppas–Sahlin | (5) | [158] |
| Multilayer CS–alginate-coated nanocarrier | Gallagher–Corrigan | (9) | [154] | |
| Alginate–carboxymethylcellulose microparticles with CS shells | Gallagher–Corrigan | (9) | [152] | |
| Diffusion and swelling | CS–alginate | Hopfenberg | (8) | [159] |
| Diffusion, erosion | DOX-loaded PLGA-QCS ii core–shell polymersomes | Korsmeyer-Peppas | (3) | [160] |
| Kopcha | (6) | |||
| PLGA/CS microcapsules | Baker–Lonsdale | (7) | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, J.; Durand, A.; Desobry, S. Chitosan-Based Particulate Carriers: Structure, Production and Corresponding Controlled Release. Pharmaceutics 2023, 15, 1455. https://doi.org/10.3390/pharmaceutics15051455
Weng J, Durand A, Desobry S. Chitosan-Based Particulate Carriers: Structure, Production and Corresponding Controlled Release. Pharmaceutics. 2023; 15(5):1455. https://doi.org/10.3390/pharmaceutics15051455
Chicago/Turabian StyleWeng, Jiaqi, Alain Durand, and Stéphane Desobry. 2023. "Chitosan-Based Particulate Carriers: Structure, Production and Corresponding Controlled Release" Pharmaceutics 15, no. 5: 1455. https://doi.org/10.3390/pharmaceutics15051455
APA StyleWeng, J., Durand, A., & Desobry, S. (2023). Chitosan-Based Particulate Carriers: Structure, Production and Corresponding Controlled Release. Pharmaceutics, 15(5), 1455. https://doi.org/10.3390/pharmaceutics15051455