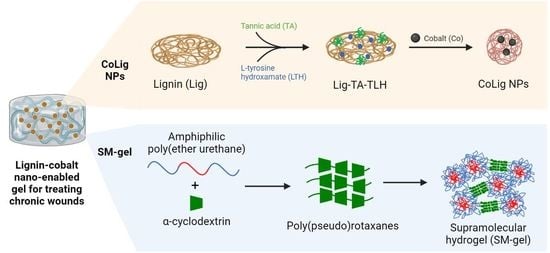
Lignin–Cobalt Nano-Enabled Poly(pseudo)rotaxane Supramolecular Hydrogel for Treating Chronic Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Materials and Cells
2.2. Enzymatic Functionalization of Lignin
2.3. Characterization of Enzymatically Functionalized Lignin (Lig-TA-LTH)
2.3.1. Attenuated Total Reflectance–Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.3.2. Measurement of the Phenolic Content
2.3.3. Quantification of the LTH Content
2.4. Preparation of Cobalt/Lignin Nanoparticles (CoLig NPs)
2.5. Characterization of Cobalt/Lignin Nanoparticles (CoLig NPs)
2.5.1. Morphological Characterization and Cobalt Loading
2.5.2. MPO and MMP Inhibition Assays
2.5.3. Antibacterial Activity of CoLig NPs
2.5.4. Cytotoxicity of CoLig NPs
2.6. Preparation and Characterization of Supramolecular Hydrogel (SM-Gel) Embedded with CoLig NPs
2.6.1. Synthesis of Hydrogel Constituent Polymers
2.6.2. Preparation of the SM-Gel Loaded with CoLig NPs
2.6.3. Bovine Serum Albumin (BSA) Uptake in the SM-Gel
2.6.4. Stability of the SM-Gel Loaded with CoLig NPs
2.6.5. Rheological Tests
2.6.6. CryoSEM
2.6.7. CoLig NPs Interaction with SHF68
2.6.8. CoLig NPs’ Release from the SM-Gel
2.6.9. Antibacterial Activity of the SM-Gel Containing CoLig NPs
2.6.10. Cytocompatibility of the SM-Gel Containing CoLig NPs
2.7. Statistical Analysis
3. Results and Discussion
3.1. CoLig NPs’ Synthesis and Characterization
3.2. MPO and MMP Inhibitory Capacity of CoLig NPs
3.3. CoLig NPs’ Antibacterial Properties and Cytotoxicity
3.4. Design of the Nano-Enabled SM-Gel
3.5. CoLig NPs’ Integration in the SM-Gel and Characterization
3.6. CoLig NPs’ Release from the SM-Gel
3.7. Antibacterial Activity and Cytocompatibility of the SM-Gel Loaded with CoLig NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robson, M.C.; Steed, D.L.; Franz, M.G. Wound Healing: Biologic Features and Approaches to Maximize Healing Trajectories. Curr. Probl. Surg. 2001, 38, A1–A140. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Phillips, T.J. Wound Healing and Treating Wounds: Differential Diagnosis and Evaluation of Chronic Wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound Microbiology and Associated Approaches to Wound Management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of Chronic Wounds in the General Population: Systematic Review and Meta-Analysis of Observational Studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef]
- Graves, N.; Phillips, C.J.; Harding, K. A Narrative Review of the Epidemiology and Economics of Chronic Wounds. Br. J. Dermatol. 2022, 187, 141–148. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [Green Version]
- Leaper, D.; Assadian, O.; Edmiston, C.E. Approach to Chronic Wound Infections. Br. J. Dermatol. 2015, 173, 351–358. [Google Scholar] [CrossRef]
- Diegelmann, R.F. Wound Healing: An Overview of Acute, Fibrotic and Delayed Healing. Front. Biosci. 2004, 9, 283. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Fu, X.; Kassim, S.Y.; Parks, W.C.; Heinecke, J.W. Hypochlorous Acid Oxygenates the Cysteine Switch Domain of Pro-Matrilysin (MMP-7): A Mechanism for Matrix Metalloproteinase Activation and Atherosclerotic Plaque Rupture by Myeloperoxidase. J. Biol. Chem. 2001, 276, 41279–41287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nève, J.; Parij, N.; Moguilevsky, N. Inhibition of the Myeloperoxidase Chlorinating Activity by Non-Steroidal Anti-Inflammatory Drugs Investigated with a Human Recombinant Enzyme. Eur. J. Pharmacol. 2001, 417, 37–43. [Google Scholar] [CrossRef]
- Nwomeh, B.C.; Yager, D.R.; Cohen, I.K. Physiology of the Chronic Wound. Clin. Plast. Surg. 1998, 25, 341–356. [Google Scholar] [CrossRef]
- Trengove, N.J.; Stacey, M.C.; Macauley, S.; Bennett, N.; Gibson, J.; Burslem, F.; Murphy, G.; Schultz, G. Analysis of the Acute and Chronic Wound Environments: The Role of Proteases and Their Inhibitors. Wound Repair Regen. 1999, 7, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, J.L.; Izzo, V.; Meaume, S.; Davies, A.H.; Lobmann, R.; Uccioli, L. Elevated Levels of Matrix Metalloproteinases and Chronic Wound Healing: An Updated Review of Clinical Evidence. J. Wound Care 2016, 25, 277–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound Healing and Treating Wounds: Chronic Wound Care and Management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Holmes, C.M. Updates on Diabetic Foot and Charcot Osteopathic Arthropathy. Curr. Diab. Rep 2018, 18, 74. [Google Scholar] [CrossRef]
- Wu, L.; Norman, G.; Dumville, J.C.; O’Meara, S.; Bell-Syer, S.E. Dressings for Treating Foot Ulcers in People with Diabetes: An Overview of Systematic Reviews. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Laurano, R.; Boffito, M.; Ciardelli, G.; Chiono, V. Wound Dressing Products: A Translational Investigation from the Bench to the Market. Eng. Regen. 2022, 3, 182–200. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, S.; Zhao, Y.; Zhai, X.; Wang, L.; Luo, H.; Xu, Z.; Dong, W.; Wu, B.; Wei, W. A Dual-Crosslinked Hydrogel Based on Gelatin Methacryloyl and Sulfhydrylated Chitosan for Promoting Wound Healing. Int. J. Mol. Sci. 2023, 24, 2447. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, H.; Pan, X.; Zhang, C.; Zhang, K.; Chen, Z.; Dong, W.; Xie, A.; Qi, X. Dendritic Hydrogels with Robust Inherent Antibacterial Properties for Promoting Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 11144–11155. [Google Scholar] [CrossRef]
- Ding, X.; Wang, Y. Weak Bond-Based Injectable and Stimuli Responsive Hydrogels for Biomedical Applications. J. Mater. Chem. B 2017, 5, 887–906. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Cai, L.; Jia, Y.-G.; Liu, S.; Chen, Y.; Ren, L. Progress in Self-Healing Hydrogels Assembled by Host–Guest Interactions: Preparation and Biomedical Applications. J. Mater. Chem. B 2019, 7, 1637–1651. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Loh, X.J. Cyclodextrin-Based Supramolecular Architectures: Syntheses, Structures, and Applications for Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Zhou, Z.; Ni, X.; Leong, K.W. Formation of Supramolecular Hydrogels Induced by Inclusion Complexation between Pluronics and α-Cyclodextrin. Macromolecules 2001, 34, 7236–7237. [Google Scholar] [CrossRef]
- Ni, X.; Cheng, A.; Li, J. Supramolecular Hydrogels Based on Self-Assembly between PEO-PPO-PEO Triblock Copolymers and α-Cyclodextrin. J. Biomed. Mater. Res. Part A 2009, 88, 1031–1036. [Google Scholar] [CrossRef]
- Li, J.; Harada, A.; Kamachi, M. Sol–Gel Transition during Inclusion Complex Formation between α-Cyclodextrin and High Molecular Weight Poly(Ethylene Glycol)s in Aqueous Solution. Polym. J. 1994, 26, 1019–1026. [Google Scholar] [CrossRef]
- Li, J.; Ni, X.; Leong, K.W. Injectable Drug-Delivery Systems Based on Supramolecular Hydrogels Formed by Poly(Ethylene Oxide)s and α-Cyclodextrin. J. Biomed. Mater. Res. Part A 2003, 65, 196–202. [Google Scholar] [CrossRef]
- Domiński, A.; Konieczny, T.; Kurcok, P. α-Cyclodextrin-Based Polypseudorotaxane Hydrogels. Materials 2020, 13, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torchio, A.; Boffito, M.; Gallina, A.; Lavella, M.; Cassino, C.; Ciardelli, G. Supramolecular Hydrogels Based on Custom-Made Poly(Ether Urethane)s and Cyclodextrins as Potential Drug Delivery Vehicles: Design and Characterization. J. Mater. Chem. B 2020, 8, 7696–7712. [Google Scholar] [CrossRef]
- Torchio, A.; Cassino, C.; Lavella, M.; Gallina, A.; Stefani, A.; Boffito, M.; Ciardelli, G. Injectable Supramolecular Hydrogels Based on Custom-Made Poly(Ether Urethane)s and α-Cyclodextrins as Efficient Delivery Vehicles of Curcumin. Mater. Sci. Eng. C 2021, 127, 112194. [Google Scholar] [CrossRef]
- Coccia, F.; Tonucci, L.; Bosco, D.; Bressan, M.; d’Alessandro, N. One-Pot Synthesis of Lignin-Stabilised Platinum and Palladium Nanoparticles and Their Catalytic Behaviour in Oxidation and Reduction Reactions. Green Chem. 2012, 14, 1073–1078. [Google Scholar] [CrossRef]
- Hu, S.; Hsieh, Y.-L. Silver Nanoparticle Synthesis Using Lignin as Reducing and Capping Agents: A Kinetic and Mechanistic Study. Int. J. Biol. Macromol. 2016, 82, 856–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morena, A.G.; Stefanov, I.; Ivanova, K.; Pérez-Rafael, S.; Sánchez-Soto, M.; Tzanov, T. Antibacterial Polyurethane Foams with Incorporated Lignin-Capped Silver Nanoparticles for Chronic Wound Treatment. Ind. Eng. Chem. Res. 2020, 59, 4504–4514. [Google Scholar] [CrossRef]
- Cheng, X.-C.; Wang, R.-L.; Dong, Z.-K.; Li, J.; Li, Y.-Y.; Li, R.-R. Design, Synthesis and Evaluation of Novel Metalloproteinase Inhibitors Based on l-Tyrosine Scaffold. Bioorg. Med. Chem. 2012, 20, 5738–5744. [Google Scholar] [CrossRef] [PubMed]
- Holms, J.; Mast, K.; Marcotte, P.; Elmore, I.; Li, J.; Pease, L.; Glaser, K.; Morgan, D.; Michaelides, M.; Davidsen, S. Discovery of Selective Hydroxamic Acid Inhibitors of Tumor Necrosis Factor-α Converting Enzyme. Bioorg. Med. Chem. Lett. 2001, 11, 2907–2910. [Google Scholar] [CrossRef]
- Morena, A.G.; Bassegoda, A.; Natan, M.; Jacobi, G.; Banin, E.; Tzanov, T. Antibacterial Properties and Mechanisms of Action of Sonoenzymatically Synthesized Lignin-Based Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 37270–37279. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef] [PubMed]
- Sterling, J.P. Silver-Resistance, Allergy, and Blue Skin: Truth or Urban Legend? Burns 2014, 40, S19–S23. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, C.T.; Raji, P. Effect of Annealing Temperature on Antibacterial, Antifungal and Structural Properties of Bio-Synthesized Co3O4 Nanoparticles Using Hibiscus Rosa-Sinensis. Mater. Res. Express 2019, 6, 095063. [Google Scholar] [CrossRef]
- Anupong, W.; On-uma, R.; Jutamas, K.; Joshi, D.; Salmen, S.H.; Alahmadi, T.A.; Jhanani, G.K. Cobalt Nanoparticles Synthesizing Potential of Orange Peel Aqueous Extract and Their Antimicrobial and Antioxidant Activity. Environ. Res. 2023, 216, 114594. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Sustainable Synthesis of Cobalt and Cobalt Oxide Nanoparticles and Their Catalytic and Biomedical Applications. Green Chem. 2020, 22, 2643–2661. [Google Scholar] [CrossRef]
- Boffito, M.; Gioffredi, E.; Chiono, V.; Calzone, S.; Ranzato, E.; Martinotti, S.; Ciardelli, G. Novel Polyurethane-Based Thermosensitive Hydrogels as Drug Release and Tissue Engineering Platforms: Design and in Vitro Characterization. Polym. Int. 2016, 65, 756–769. [Google Scholar] [CrossRef]
- Morena, A.G.; Bassegoda, A.; Hoyo, J.; Tzanov, T. Hybrid Tellurium–Lignin Nanoparticles with Enhanced Antibacterial Properties. ACS Appl. Mater. Interfaces 2021, 13, 14885–14893. [Google Scholar] [CrossRef]
- Boffito, M.; Torchio, A.; Tonda-Turo, C.; Laurano, R.; Gisbert-Garzarán, M.; Berkmann, J.C.; Cassino, C.; Manzano, M.; Duda, G.N.; Vallet-Regí, M.; et al. Hybrid Injectable Sol-Gel Systems Based on Thermo-Sensitive Polyurethane Hydrogels Carrying PH-Sensitive Mesoporous Silica Nanoparticles for the Controlled and Triggered Release of Therapeutic Agents. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Soyocak, A.; Kurt, H.; Cosan, D.T.; Saydam, F.; Calis, I.; Kolac, U.; Koroglu, Z.O.; Degirmenci, I.; Mutlu, F.S.; Gunes, H. Tannic Acid Exhibits Anti-Inflammatory Effects on Formalin-Induced Paw Edema Model of Inflammation in Rats. Hum. Exp. Toxicol. 2019, 38, 1296–1301. [Google Scholar] [CrossRef]
- Perelshtein, I.; Ruderman, E.; Francesko, A.; Fernandes, M.M.; Tzanov, T.; Gedanken, A. Tannic Acid NPs—Synthesis and Immobilization onto a Solid Surface in a One-Step Process and Their Antibacterial and Anti-Inflammatory Properties. Ultrason. Sonochem. 2014, 21, 1916–1920. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.P. Hydroxamic Acids as Matrix Metalloproteinase Inhibitors. In Matrix Metalloproteinase Inhibitors; Gupta, S.P., Ed.; Experientia Supplementum; Springer: Basel, Switzerland, 2012; Volume 103, pp. 137–176. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.; Gu, L.; Wu, W.; Zhao, H.; Jin, Y. Structural Elucidation and Antioxidant Activity of Lignin Isolated from Rice Straw and Alkali-oxygen Black Liquor. Int. J. Biol. Macromol. 2018, 116, 513–519. [Google Scholar] [CrossRef]
- Jiang, P.; Sheng, X.; Yu, S.; Li, H.; Lu, J.; Zhou, J.; Wang, H. Preparation and Characterization of Thermo-Sensitive Gel with Phenolated Alkali Lignin. Sci. Rep. 2018, 8, 14450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, B.; Jia, Z.; Jiang, H.; Wang, S.; Min, D. Improving the Reactivity of Sugarcane Bagasse Kraft Lignin by a Combination of Fractionation and Phenolation for Phenol–Formaldehyde Adhesive Applications. Polymers 2020, 12, 1825. [Google Scholar] [CrossRef]
- Momić, T.; Vujčić, Z.; Vasić, V. Kinetics of Inhibition of Peroxidase Activity of Myeloperoxidase by Quercetin. Int. J. Chem. Kinet. 2008, 40, 384–394. [Google Scholar] [CrossRef]
- Mota, F.A.R.; Pereira, S.A.P.; Araújo, A.R.T.S.; Gullón, B.; Passos, M.L.C.; Saraiva, M.L.M.F.S. Automatic Identification of Myeloperoxidase Natural Inhibitors in Plant Extracts. Molecules 2022, 27, 1825. [Google Scholar] [CrossRef]
- Díaz-GonzáLez, M.; Rocasalbas, G.; Francesko, A.; Touriño, S.; Torres, J.L.; Tzanov, T. Inhibition of Deleterious Chronic Wound Enzymes with Plant Polyphenols. Biocatal. Biotransform. 2012, 30, 102–110. [Google Scholar] [CrossRef]
- Heffern, M.C.; Yamamoto, N.; Holbrook, R.J.; Eckermann, A.L.; Meade, T.J. Cobalt Derivatives as Promising Therapeutic Agents. Curr. Opin. Chem. Biol. 2013, 17, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhang, B.; Yan, B. Regulation of Enzyme Activity through Interactions with Nanoparticles. Int. J. Mol. Sci. 2009, 10, 4198–4209. [Google Scholar] [CrossRef] [Green Version]
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.-P. Biofilms in Diabetic Foot Ulcers: Significance and Clinical Relevance. Microorganisms 2020, 8, 1580. [Google Scholar] [CrossRef]
- Mottola, C.; Mendes, J.J.; Cristino, J.M.; Cavaco-Silva, P.; Tavares, L.; Oliveira, M. Polymicrobial Biofilms by Diabetic Foot Clinical Isolates. Folia Microbiol. 2016, 61, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and Antioxidant Activities of Lignin from Residue of Corn Stover to Ethanol Production. Ind. Crops Prod. 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium Alginate Hydrogel Containing Platelet-Rich Plasma for Wound Healing. Colloids Surf. B Biointerfaces 2023, 222, 113096. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yao, J.; Zhao, J.; Wang, S. Injectable Wound Dressing Based on Carboxymethyl Chitosan Triple-Network Hydrogel for Effective Wound Antibacterial and Hemostasis. Int. J. Biol. Macromol. 2023, 225, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Fleury, C.; Petit, A.; Mwale, F.; Antoniou, J.; Zukor, D.J.; Tabrizian, M.; Huk, O.L. Effect of Cobalt and Chromium Ions on Human MG-63 Osteoblasts in Vitro: Morphology, Cytotoxicity, and Oxidative Stress. Biomaterials 2006, 27, 3351–3360. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Y, A.O.S.; Ahamed, M.; Siddiqui, M.A.; Al-Khedhairy, A.A. Oxidative Stress Contributes to Cobalt Oxide Nanoparticles-Induced Cytotoxicity and DNA Damage in Human Hepatocarcinoma Cells. Int. J. Nanomed. 2013, 8, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Cutting, K.F. Wound Exudate: Composition and Functions. Br. J. Community Nurs. 2003, 8, S4–S9. [Google Scholar] [CrossRef]
- Adderley, U.J. Managing Wound Exudate and Promoting Healing. Br. J. Community Nurs. 2010, 15, S15–S20. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and Pathophysiology of Matrix Metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Rafael, S.; Ivanova, K.; Stefanov, I.; Puiggalí, J.; del Valle, L.J.; Todorova, K.; Dimitrov, P.; Hinojosa-Caballero, D.; Tzanov, T. Nanoparticle-Driven Self-Assembling Injectable Hydrogels Provide a Multi-Factorial Approach for Chronic Wound Treatment. Acta Biomater. 2021. [Google Scholar] [CrossRef]
- Morena, A.G.; Pérez-Rafael, S.; Tzanov, T. Lignin-Based Nanoparticles as Both Structural and Active Elements in Self-Assembling and Self-Healing Multifunctional Hydrogels for Chronic Wound Management. Pharmaceutics 2022, 14, 2658. [Google Scholar] [CrossRef] [PubMed]
- Marmorat, C.; Arinstein, A.; Koifman, N.; Talmon, Y.; Zussman, E.; Rafailovich, M. Cryo-Imaging of Hydrogels Supermolecular Structure. Sci. Rep. 2016, 6, 25495. [Google Scholar] [CrossRef]
- Kaberova, Z.; Karpushkin, E.; Nevoralová, M.; Vetrík, M.; Šlouf, M.; Dušková-Smrčková, M. Microscopic Structure of Swollen Hydrogels by Scanning Electron and Light Microscopies: Artifacts and Reality. Polymers 2020, 12, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, R.; Matsushita, Y.; Umemoto, K.; Usuki, A.; Fukushima, K. Enzymatic Polymerization of Coniferyl Alcohol in the Presence of Cyclodextrins. Biomacromolecules 2006, 7, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Lawson, K.R.; Perkins, J.; Urch, C.J. Aromatic Interactions. J. Chem. Soc. Perkin Trans. 2001, 2, 651–669. [Google Scholar] [CrossRef]
- Paarakh, M.P.; Jose, P.A.; Setty, C.; Peter, G.V. Release Kinetics—Concepts and Applications. IJPRT 2018, 8, 12–20. [Google Scholar]
- Laurano, R.; Chiono, V.; Ceresa, C.; Fracchia, L.; Zoso, A.; Ciardelli, G.; Boffito, M. Custom-Design of Intrinsically Antimicrobial Polyurethane Hydrogels as Multifunctional Injectable Delivery Systems for Mini-Invasive Wound Treatment. Eng. Regen. 2021, 2, 263–278. [Google Scholar] [CrossRef]














| Technique | Size | PDI | ζ Potential |
|---|---|---|---|
| DLS | 192 ± 1 nm | 0.22 ± 0.01 | −23.1 ± 0.8 mV |
| NTA | 107 ± 38 nm | - | - |
| SEM | 47 ± 7 nm | - | - |
| S. aureus | P. aeruginosa | |
|---|---|---|
| CoLig NPs | 1.2 | 2.4 |
| Lig NPs | 3.3 | 3.3 |
| G′ LVE (Pa) | G″ LVE (Pa) | Tf (%) | G′ Recovered (%) after 3 Cyclic Ruptures | |
|---|---|---|---|---|
| SM-gel | 4551.2 | 400.8 | 21.4 | 89% |
| SM-gel + CoLig NPs | 7114.1 | 861.9 | 13.6 | 87% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crivello, G.; Orlandini, G.; Morena, A.G.; Torchio, A.; Mattu, C.; Boffito, M.; Tzanov, T.; Ciardelli, G. Lignin–Cobalt Nano-Enabled Poly(pseudo)rotaxane Supramolecular Hydrogel for Treating Chronic Wounds. Pharmaceutics 2023, 15, 1717. https://doi.org/10.3390/pharmaceutics15061717
Crivello G, Orlandini G, Morena AG, Torchio A, Mattu C, Boffito M, Tzanov T, Ciardelli G. Lignin–Cobalt Nano-Enabled Poly(pseudo)rotaxane Supramolecular Hydrogel for Treating Chronic Wounds. Pharmaceutics. 2023; 15(6):1717. https://doi.org/10.3390/pharmaceutics15061717
Chicago/Turabian StyleCrivello, Giulia, Giuliana Orlandini, Angela Gala Morena, Alessandro Torchio, Clara Mattu, Monica Boffito, Tzanko Tzanov, and Gianluca Ciardelli. 2023. "Lignin–Cobalt Nano-Enabled Poly(pseudo)rotaxane Supramolecular Hydrogel for Treating Chronic Wounds" Pharmaceutics 15, no. 6: 1717. https://doi.org/10.3390/pharmaceutics15061717
APA StyleCrivello, G., Orlandini, G., Morena, A. G., Torchio, A., Mattu, C., Boffito, M., Tzanov, T., & Ciardelli, G. (2023). Lignin–Cobalt Nano-Enabled Poly(pseudo)rotaxane Supramolecular Hydrogel for Treating Chronic Wounds. Pharmaceutics, 15(6), 1717. https://doi.org/10.3390/pharmaceutics15061717