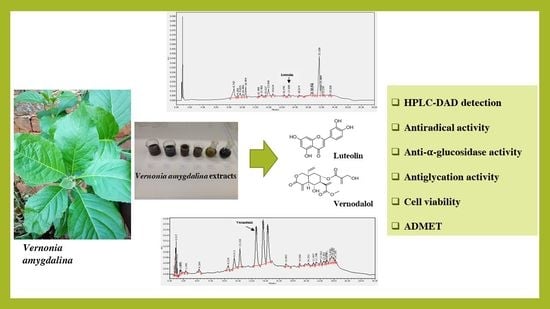
Luteolin and Vernodalol as Bioactive Compounds of Leaf and Root Vernonia amygdalina Extracts: Effects on α-Glucosidase, Glycation, ROS, Cell Viability, and In Silico ADMET Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Vernonia amygdalina Extracts
2.3. HPLC LC/DAD Analysis
2.4. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.5. α-Glucosidase Inhibition Assay
2.6. Protein Glycation Inhibition
2.7. Cell Viability Assay
2.8. Prediction of Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) Properties
2.9. Statistical Analysis
3. Results and Discussion
3.1. Measurements of Luteolin and Vernodalol in Leaf and Root Vernonia amygdalina Extracts
3.2. Antiradical Activity
3.3. α-Glucosidase Activity
3.4. Antiglycation Activity
3.5. HT-29 Cell Viability
3.6. In Silico Prediction of ADMET Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- WHO Report. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019.
- Odeyemi, S.; Bradley, G. Medicinal plants used for the traditional management of diabetes in the Eastern Cape, South Africa: Pharmacology and toxicology. Molecules 2018, 23, 2759. [Google Scholar] [CrossRef]
- Ogidi, O.I.; George, D.G.; Esie, N.G. Ethnopharmacological properties of Vernonia amygdalina (Bitter Leave) medicinal plant. J. Med. Plants 2019, 7, 175–181. [Google Scholar]
- Toyang, N.J.; Verpoorte, R. A review of the medicinal potentials of plants of the genus Vernonia (Asteraceae). J. Ethnopharmacol. 2013, 146, 681–723. [Google Scholar] [CrossRef] [PubMed]
- Medjiofack Djeujo, F.; Cusinato, F.; Ragazzi, E.; Froldi, G. α-Glucosidase and advanced glycation end products inhibition with Vernonia amygdalina root and leaf extracts: New data supporting the antidiabetic properties. J. Pharm. Pharmacol. 2021, 73, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Atangwho, I.J.; Egbung, G.E.; Ahmad, M.; Yam, M.F.; Asmawi, M.Z. Antioxidant versus anti-diabetic properties of leaves from Vernonia amygdalina Del. growing in Malaysia. Food Chem. 2013, 141, 3428–3434. [Google Scholar] [CrossRef]
- Michael, U.A.; David, B.U.; Theophine, C.O.; Philip, F.U.; Ogochukwu, A.M.; Benson, V.A. Antidiabetic effect of combined aqueous leaf extract of Vernonia amygdalina and metformin in rats. J. Basic Clin. Pharm. 2010, 1, 197–202. [Google Scholar]
- Nwanjo, H.U. Efficacy of aqueous leaf extract of Vernonia amygdalina on plasma lipoprotein and oxidative status in diabetic rat models. Niger. J. Physiol. Sci. Off. Publ. Physiol. Soc. Niger. 2005, 20, 39–42. [Google Scholar]
- Okon, U.A.; Umoren, I.U. Comparison of antioxidant activity of insulin, Ocimum gratissimum L., and Vernonia amygdalina L. in type 1 diabetic rat model. J. Integr. Med. 2017, 15, 302–309. [Google Scholar] [CrossRef]
- Asante, D.B.; Effah-Yeboah, E.; Barnes, P.; Abban, H.A.; Ameyaw, E.O.; Boampong, J.N.; Ofori, E.G.; Dadzie, J.B. Antidiabetic Effect of Young and Old Ethanolic Leaf Extracts of Vernonia amygdalina: A Comparative Study. J. Diabetes Res. 2016, 2016, 8252741, Erratum in J. Diabetes Res. 2017, 2017, 5618548. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Song, L.; Huang, D.; Tan, B.K.H. Polyphenols-rich Vernonia amygdalina shows anti-diabetic effects in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2011, 133, 598–607. [Google Scholar] [CrossRef]
- Atangwho, I.J.; Yin, K.B.; Umar, M.I.; Ahmad, M.; Asmawi, M.Z. Vernonia amygdalina simultaneously suppresses gluconeogenesis and potentiates glucose oxidation via the pentose phosphate pathway in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2014, 14, 426. [Google Scholar] [CrossRef]
- Akerele, G.P.; Adedayo, B.C.; Oboh, G.; Ademosun, A.O.; Oyeleye, S.I. Glycemic indices and effect of bitter leaf (Vernonia amygdalina) flavored non-alcoholic wheat beer (NAWB) on key carbohydrate metabolizing enzymes in high fat diet fed (HFD)/STZ- induced diabetic Wistar rats. J. Food Front. 2022, 46, e14511. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Mudalip, S.K.A.; Olalere, O.A. Phytochemical and Pharmacological Properties of Vernonia amygdalina: A Review. J. Chem. Eng. Ind. Biotechnol. 2017, 2, 80–96. [Google Scholar] [CrossRef]
- Dumas, N.G.E.; Anderson, N.T.Y.; Godswill, N.N.; Thiruvengadam, M.; Ana-Maria, G.; Ramona, P.; Crisan, G.C.; Laurian, V.; Shariati, M.A.; Tokhtarov, Z.; et al. Secondary metabolite contents and antimicrobial activity of leaf extracts reveal genetic variability of Vernonia amygdalina and Vernonia calvoana morphotypes. Biotechnol. Appl. Biochem. 2021, 68, 938–947. [Google Scholar] [CrossRef]
- Do Socorro Chagas, M.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-De-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxidative Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.H.; Ha, S.E.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Park, K.I.; Heo, J.D.; Kim, H.W.; Kim, G.S. Flavones: Six Selected Flavones and Their Related Signaling Pathways That Induce Apoptosis in Cancer. Int. J. Mol. Sci. 2022, 23, 10965. [Google Scholar] [CrossRef]
- Mahdiani, S.; Omidkhoda, N.; Heidari, S.; Hayes, A.W.; Karimi, G. Protective effect of luteolin against chemical and natural toxicants by targeting NF-κB pathway. BioFactors 2022, 48, 744–762. [Google Scholar] [CrossRef]
- Vonia, S.; Hartati, R.; Insanu, M. In Vitro Alpha-Glucosidase Inhibitory Activity and the Isolation of Luteolin from the Flower of Gymnanthemum amygdalinum (Delile) Sch. Bip ex Walp. Molecules 2022, 27, 2132. [Google Scholar] [CrossRef]
- Li, B.L.; Zhao, D.Y.; Du, P.L.; Wang, X.T.; Yang, Q.; Cai, Y.R. Luteolin alleviates ulcerative colitis through SHP-1/STAT3 pathway. Inflamm. Res. 2021, 70, 705–717. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Djeujo, F.M.; Ragazzi, E.; Urettini, M.; Sauro, B.; Cichero, E.; Tonelli, M.; Froldi, G. Magnolol and Luteolin Inhibition of α-Glucosidase Activity: Kinetics and Type of Interaction Detected by In Vitro and In Silico Studies. Pharmaceuticals 2022, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, Y.; Deng, G.; Ma, L.; Wei, G.; Zheng, G.; Han, X.; He, D.; Zhao, Y.; He, J.; et al. Vernodalol enhances TRAIL-induced apoptosis in diffuse large B-cell lymphoma cells. Mol. Carcinog. 2017, 56, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Sinisi, A.; Millán, E.; Abay, S.M.; Habluetzel, A.; Appendino, G.; Muñoz, E.; Taglialatela-Scafati, O. Poly-Electrophilic Sesquiterpene Lactones from Vernonia amygdalina: New Members and Differences in Their Mechanism of Thiol Trapping and in Bioactivity. J. Nat. Prod. 2015, 78, 1618–1623. [Google Scholar] [CrossRef]
- Kimani, N.M.; Matasyoh, J.C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Sesquiterpene lactones from Vernonia cinerascens Sch. Bip. and their in vitro antitrypanosomal activity. Molecules 2018, 23, 248. [Google Scholar] [CrossRef]
- Pedersen, M.M.; Chukwujekwu, J.C.; Lategan, C.A.; van Staden, J.; Smith, P.J.; Staerk, D. Antimalarial sesquiterpene lactones from Distephanus angulifolius. Phytochemistry 2009, 70, 601–607. [Google Scholar] [CrossRef]
- Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Liang, W.S.; Ky, H.; Yousr, A.H.N.; Alitheen, N.B. Vernonia amygdalina, an ethnoveterinary and ethnomedical used green vegetable with multiple bioactivities. J. Med. Plants Res. 2010, 4, 2787–2812. [Google Scholar]
- Erasto, P.; Grierson, D.S.; Afolayan, A.J. Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina. J. Ethnopharmacol. 2006, 106, 117–120. [Google Scholar] [CrossRef]
- Kraft, C.; Jenett-Siems, K.; Siems, K.; Jakupovic, J.; Mavi, S.; Bienzle, U.; Eich, E. In vitro antiplasmodial evaluation of medicinal plants from Zimbabwe. Phytother. Res. 2003, 17, 123–128. [Google Scholar] [CrossRef]
- Wu, W.; Han, X.; Wu, C.; Wei, G.; Zheng, G.; Li, Y.; Yang, Y.; Yang, L.; He, D.; Zhao, Y.; et al. Vernodalol mediates antitumor effects in acute promyelocytic leukemia cells. Oncol. Lett. 2018, 15, 2227–2235. [Google Scholar] [CrossRef]
- Nerdy, N.; Lestari, P.; Fahdi, F.; Putra, E.D.L.; Amir, S.A.B.; Yusuf, F.; Bakri, T.K. In Silico Studies of Sesquiterpene Lactones from Vernonia amygdalina Delile on the Expression of EGFR and VEGFR as a New Anticancer Potential. Pharmacogn. J. 2022, 14, 91–97. [Google Scholar] [CrossRef]
- Bindu, J.; Narendhirakannan, R.T. Role of medicinal plants in the management of diabetes mellitus: A review. 3 Biotech 2019, 9, 4. [Google Scholar] [CrossRef]
- Jayaweera, U. Diabetes and Vernonia amygdalina Delile (Asteraceae). Biointerface Res. Appl. Chem. 2022, 12, 4496–4517. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Djeujo, F.M.; Francesconi, V.; Gonella, M.; Ragazzi, E.; Tonelli, M.; Froldi, G. Anti-alpha-glucosidase and antiglycation Activities of alpha-Mangostin and new xanthenone derivatives: Kinetics and mechanistic insights through in vitro studies. Molecules 2022, 27, 547. [Google Scholar] [CrossRef]
- Mou, L.; Hu, P.; Cao, X.; Chen, Y.; Xu, Y.; He, T.; Wei, Y.; He, R. Comparison of bovine serum albumin glycation by ribose and fructose in vitro and in vivo. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166283. [Google Scholar] [CrossRef]
- Froldi, G.; Djeujo, F.M.; Bulf, N.; Caparelli, E.; Ragazzi, E. Comparative Evaluation of the Antiglycation and Anti-α-Glucosidase Activities of Baicalein, Baicalin (Baicalein 7-O-Glucuronide) and the Antidiabetic Drug Metformin. Pharmaceutics 2022, 14, 2141. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay BT—Cancer Cell Culture: Methods and Protocols. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef] [PubMed]
- ACD/Labs The Platform for In Silico Molecular Property Calculations. Available online: https://www.acdlabs.com/products/percepta-platform/ (accessed on 28 February 2023).
- National Library of Medicine. PubChem Database. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 15 April 2023).
- Igile, G.O.; Oleszek, W.; Jurzysta, M.; Burda, S.; Fafunso, M.; Fasanmade, A.A. Flavonoids from Vernonia amygdalina and Their Antioxidant Activities. J. Agric. Food Chem. 1994, 42, 2445–2448. [Google Scholar] [CrossRef]
- Syahputra, R.A.; Harahap, U.; Dalimunthe, A.; Pandapotan, M.; Satria, D. Protective effect of Vernonia amygdalina Delile against doxorubicin-induced cardiotoxicity. Heliyon 2021, 7, e07434. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Zhao, W.; Xu, S.; Weng, J. Resveratrol in Treating Diabetes and Its Cardiovascular Complications: A Review of Its Mechanisms of Action. Antioxidants 2022, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Testa, R.; Genovese, S. Nutrition, Metabolism & Cardiovascular Diseases Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 285–292. [Google Scholar] [CrossRef]
- Obi, B.C.; Okoye, T.C.; Okpashi, V.E.; Igwe, C.N.; Alumanah, E.O. Comparative Study of the Antioxidant Effects of Metformin, Glibenclamide, and Repaglinide in Alloxan-Induced Diabetic Rats. J. Diabetes Res. 2016, 2016, 1635361. [Google Scholar] [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Biochimica et Biophysica Acta Diabetes, oxidative stress and therapeutic strategies. BBA Gen. Subj. 2014, 1840, 2709–2729. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced glycation end products and diabetes mellitus: Mechanisms and perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Wu, C.H.; Yen, G.C. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J. Agric. Food Chem. 2005, 53, 3167–3173. [Google Scholar] [CrossRef]
- Muramatsu, D.; Uchiyama, H.; Kida, H.; Iwai, A. Cell cytotoxity and anti-glycation activity of taxifolin-rich extract from Japanese larch, Larix kaempferi. Heliyon 2019, 5, e02047. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Wang, T.T.; Wang, S.K.; Huang, G.L.; Sun, G.J. Luteolin Induced-growth inhibition and apoptosis of human esophageal squamous carcinoma cell line Eca109 cells in vitro. Asian Pac. J. Cancer Prev. 2012, 13, 5455–5461. [Google Scholar] [CrossRef]
- Cai, X.; Ye, T.; Liu, C.; Lu, W.; Lu, M.; Zhang, J.; Wang, M.; Cao, P. Luteolin induced G2 phase cell cycle arrest and apoptosis on non-small cell lung cancer cells. Toxicol. Vitr. 2011, 25, 1385–1391. [Google Scholar] [CrossRef]
- Wang, F.; Gao, F.; Pan, S.; Zhao, S.; Xue, Y. Luteolin induces apoptosis, G0/G1 cell cycle growth arrest and mitochondrial membrane potential loss in neuroblastoma brain tumor cells. Drug Res. 2015, 65, 91–95. [Google Scholar] [CrossRef]
- Abbasi, N.; Khosravi, A.; Aidy, A.; Shafiei, M. Biphasic response to luteolin in MG-63 osteoblast-like cells under high glucose-induced oxidative stress. Iran. J. Med. Sci. 2016, 41, 118–125. [Google Scholar]
- Sonoda, M.; Nishiyama, T.; Matsukawa, Y.; Moriyasu, M. Cytotoxic activities of flavonoids from two Scutellaria plants in Chinese medicine. J. Ethnopharmacol. 2004, 91, 65–68. [Google Scholar] [CrossRef]
- Nna, V.U.; McGrowder, D.; Nwokocha, C. Nutraceutical management of metabolic syndrome as a palliative and a therapeutic to coronavirus disease (COVID) crisis. Arch. Physiol. Biochem. 2021, 1–20. [Google Scholar] [CrossRef]
- Lin, L.C.; Pai, Y.F.; Tsai, T.H. Isolation of Luteolin and Luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and Their Pharmacokinetics in Rats. J. Agric. Food Chem. 2015, 63, 7700–7706. [Google Scholar] [CrossRef]
- Cao, L.; Kwara, A.; Greenblatt, D.J. Metabolic interactions between acetaminophen (paracetamol) and two flavonoids, luteolin and quercetin, through in-vitro inhibition studies. J. Pharm. Pharmacol. 2017, 69, 1762–1772. [Google Scholar] [CrossRef]
- Quintieri, L.; Palatini, P.; Nassi, A.; Ruzza, P.; Floreani, M. Flavonoids diosmetin and luteolin inhibit midazolam metabolism by human liver microsomes and recombinant CYP 3A4 and CYP3A5 enzymes. Biochem. Pharmacol. 2008, 75, 1426–1437. [Google Scholar] [CrossRef]
- Kimura, Y.; Ito, H.; Ohnishi, R.; Hatano, T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem. Toxicol. 2010, 48, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Kaci, H.; Bodnárová, S.; Fliszár-Nyúl, E.; Lemli, B.; Pelantová, H.; Valentová, K.; Bakos, É.; Özvegy-Laczka, C.; Poór, M. Interaction of luteolin, naringenin, and their sulfate and glucuronide conjugates with human serum albumin, cytochrome P450 (CYP2C9, CYP2C19, and CYP3A4) enzymes and organic anion transporting polypeptide (OATP1B1 and OATP2B1) transporters. Biomed. Pharmacother. 2023, 157, 114078. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Y.; Sharifi-Rad, J.; Antika, G.; Yilmaz, Y.B.; Tumer, T.B.; Abuhamdah, S.; Chandra, S.; Saklani, S.; Kiliç, C.S.; Sestito, S.; et al. Paving Luteolin Therapeutic Potentialities and Agro-Food-Pharma Applications: Emphasis on in Vivo Pharmacological Effects and Bioavailability Traits. Oxidative Med. Cell. Longev. 2021, 2021, 1987588. [Google Scholar] [CrossRef] [PubMed]
- Ndip, R.N.; Tanih, N.F.; Kuete, V. 20—Antidiabetes Activity of African Medicinal Plants; Kuete, V., Ed.; Elsevier: Oxford, UK, 2013; pp. 753–786. ISBN 978-0-12-405927-6. [Google Scholar]
- Gurib-fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Tekou, F.A.; Kuate, D.; Nguekouo, P.T.; Ypolyte, C.; Julius, W.; Oben, E. Effect of cooking treatments on the phytochemical composition and antidiabetic potential of Vernonia amygdalina. Food Sci. Nutr. 2018, 6, 1684–1691. [Google Scholar] [CrossRef]
- Alhujaily, M.; Dhifi, W.; Mnif, W. An Overview of the Potential of Medicinal Plants Used in the Development of Nutraceuticals for the Management of Diabetes Mellitus: Proposed Biological Mechanisms. Processes 2022, 10, 2044. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef]







| Compound | MW (g/mol) | LogP | BBB Permeability | P-Glycoprotein Substrate | CYP Substrate | Intestinal Absorption (Human) % | VDss (Human) Log L/kg | CLtot (Log mL/min/kg) | Oral Acute Toxicity (LD50) |
|---|---|---|---|---|---|---|---|---|---|
| Luteolin (flavone) | 286.239 | 2.2824 | −0.907 | Yes | CYP1A2 CYP2C6 | 81.13 | 1.153 | 0.495 | 2.45 mol/kg rat |
| Vernodalol (terpene) | 392.404 | 0.4583 | −0.48 | Yes | No | 75.39 | −0.197 | 0.747 | 2.39 mol/kg rat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djeujo, F.M.; Stablum, V.; Pangrazzi, E.; Ragazzi, E.; Froldi, G. Luteolin and Vernodalol as Bioactive Compounds of Leaf and Root Vernonia amygdalina Extracts: Effects on α-Glucosidase, Glycation, ROS, Cell Viability, and In Silico ADMET Parameters. Pharmaceutics 2023, 15, 1541. https://doi.org/10.3390/pharmaceutics15051541
Djeujo FM, Stablum V, Pangrazzi E, Ragazzi E, Froldi G. Luteolin and Vernodalol as Bioactive Compounds of Leaf and Root Vernonia amygdalina Extracts: Effects on α-Glucosidase, Glycation, ROS, Cell Viability, and In Silico ADMET Parameters. Pharmaceutics. 2023; 15(5):1541. https://doi.org/10.3390/pharmaceutics15051541
Chicago/Turabian StyleDjeujo, Francine Medjiofack, Valentina Stablum, Elisa Pangrazzi, Eugenio Ragazzi, and Guglielmina Froldi. 2023. "Luteolin and Vernodalol as Bioactive Compounds of Leaf and Root Vernonia amygdalina Extracts: Effects on α-Glucosidase, Glycation, ROS, Cell Viability, and In Silico ADMET Parameters" Pharmaceutics 15, no. 5: 1541. https://doi.org/10.3390/pharmaceutics15051541
APA StyleDjeujo, F. M., Stablum, V., Pangrazzi, E., Ragazzi, E., & Froldi, G. (2023). Luteolin and Vernodalol as Bioactive Compounds of Leaf and Root Vernonia amygdalina Extracts: Effects on α-Glucosidase, Glycation, ROS, Cell Viability, and In Silico ADMET Parameters. Pharmaceutics, 15(5), 1541. https://doi.org/10.3390/pharmaceutics15051541